¿Alguna vez te has preguntado por qué los bolígrafos de microagujas con velocidad ajustable pueden producir resultados diferentes incluso con la misma profundidad de la aguja?
Explicación de los ajustes de velocidad del bolígrafo de microagujas
Explicaré qué significan los ajustes de velocidad del bolígrafo de microagujas, cómo afectan los resultados del tratamiento y por qué la microaguja puede ser eficaz cuando el cuidado tópico de la piel falla. Abordaré la mecánica, el razonamiento clínico, las recomendaciones prácticas, las consideraciones de seguridad y los cuidados posteriores para que, ya sea médico, técnico o paciente, pueda tomar decisiones informadas.
Introducción a los bolígrafos de microagujas
Utilizo microagujas en la práctica clínica y asesoro a mis pacientes sobre la elección de dispositivos y protocolos. Estos dispositivos se han popularizado porque producen microlesiones controladas que estimulan el colágeno y mejoran la administración del producto tópico.
En esta sección, describo la diferencia entre los dispositivos tipo pluma y otros tipos de punción, y la importancia de ajustar la velocidad. Enfatizaré la relación entre la velocidad, el número de agujas y la respuesta del tejido.
¿Qué es un bolígrafo de microagujas?
Considero que un bolígrafo de microagujas es un dispositivo portátil y motorizado que introduce repetidamente una serie de pequeñas agujas en la piel.
Explico que, a diferencia de los rodillos manuales, los bolígrafos permiten controlar la profundidad y la velocidad de la aguja, proporcionan una entrada perpendicular a la piel y reducen la fricción y el desgarro. Estas diferencias técnicas son importantes para los resultados y la seguridad.
Por qué es importante ajustar la velocidad
Describo cómo la velocidad cambia la cantidad de microlesiones creadas por segundo y altera las fuerzas de corte mecánicas, la percepción del dolor y la generación de calor.
También observo que la velocidad interactúa con la longitud de la aguja, el tipo de cartucho y la zona anatómica tratada. Las combinaciones óptimas reducen los traumatismos innecesarios y maximizan la señalización regenerativa.
Cómo funciona biológicamente la microaguja
Presentaré la base biológica de la eficacia de la microaguja, haciendo hincapié en la cascada de curación de heridas y la administración transdérmica mejorada.
Esta sección tiene como objetivo aclarar los mecanismos para que los ajustes de velocidad tengan sentido en el contexto de la respuesta del tejido.
La cascada de cicatrización de heridas y la inducción de colágeno
Explico que las microlesiones controladas inician la hemostasia, seguida de inflamación, proliferación y remodelación. Estas etapas reclutan plaquetas, neutrófilos, macrófagos, fibroblastos y células endoteliales.
Señalo que los tipos de colágeno I y III se sintetizan durante la remodelación, lo que mejora la textura y la firmeza de la piel, así como la remodelación de las cicatrices en cuestión de semanas o meses. La velocidad influye en la densidad y el patrón de las microlesiones y, por lo tanto, en la intensidad de la señalización.
Administración mejorada de agentes tópicos
Describo cómo los microcanales reducen la función de barrera del estrato córneo y permiten una mayor penetración de sueros, péptidos, factores de crecimiento y otros activos.
Enfatizo que la microaguja no es solo un método de administración, sino también un estímulo biológico. Cuando se usan agentes tópicos inmediatamente después del tratamiento, su contacto más profundo puede potenciar los resultados, siempre que se respeten las normas de esterilización y la seguridad de los ingredientes.
Por qué la microaguja funciona cuando el cuidado tópico de la piel no funciona
Con frecuencia les explico a los pacientes que los productos tópicos pueden fallar debido a una penetración limitada, estímulos biológicos insuficientes o cambios crónicos en los tejidos que necesitan un reinicio de la cicatrización de la herida.
Aquí explico las razones clave por las que la microaguja puede tener éxito cuando el cuidado de la piel por sí solo es insuficiente.
Limitaciones de barrera del cuidado tópico de la piel
Señalo que el estrato córneo restringe el paso de muchas moléculas activas, particularmente péptidos grandes, proteínas y factores de crecimiento.
Explico que incluso los productos bien formulados pueden no alcanzar la epidermis o dermis viable donde residen las células diana. La microaguja supera esta barrera creando canales físicos.
Daños crónicos y necesidad de remodelación
Analizo cómo el fotoenvejecimiento, las cicatrices del acné y la flacidez prolongada implican cambios arquitectónicos en el colágeno dérmico que los antioxidantes o retinoides tópicos no pueden revertir por completo.
Destaco que la microaguja desencadena un proceso de remodelación que reemplaza la matriz extracelular desorganizada con colágeno y elastina más nuevos, produciendo una mejora estructural en lugar de solo una modulación bioquímica.
Reclutamiento celular y liberación de factores de crecimiento local
Observo que las microlesiones reclutan células inmunes y plaquetas que liberan moléculas de señalización (factor de crecimiento transformante beta (TGF-β), factor de crecimiento derivado de plaquetas (PDGF), factor de crecimiento endotelial vascular (VEGF)), que los tópicos por sí solos rara vez inducen en la misma magnitud.
Destaco que esta orquestación local de la reparación es una ventaja fundamental de la estimulación mecánica sobre la aplicación tópica pasiva.
Componentes y parámetros del bolígrafo de microagujas
Explico los principales parámetros del dispositivo que influyen en los resultados: profundidad de la aguja, número/disposición de las agujas, material de la aguja, diseño del cartucho y velocidad.
Debajo de cada parámetro proporciono consideraciones prácticas para que entiendas cómo la velocidad encaja en el protocolo general.
Profundidad de la aguja y orientación del tejido
Explico que la profundidad es el determinante principal de qué capas de la piel están involucradas: epidermis superficial, dermis papilar o dermis reticular.
También advierto que una penetración más profunda generalmente requiere movimientos más lentos y controlados y, a menudo, velocidades más bajas para evitar desgarros y dolor innecesarios.
Diseño, recuento y configuración de cartuchos de agujas
Describo cómo los cartuchos varían en el número y disposición de las agujas, lo que cambia el área tratada por pasada y la distribución de la presión sobre la piel.
Menciono que un mayor número de agujas puede reducir la cantidad de pasadas necesarias, pero la velocidad debe ajustarse para garantizar una entrada constante y un corte mínimo.
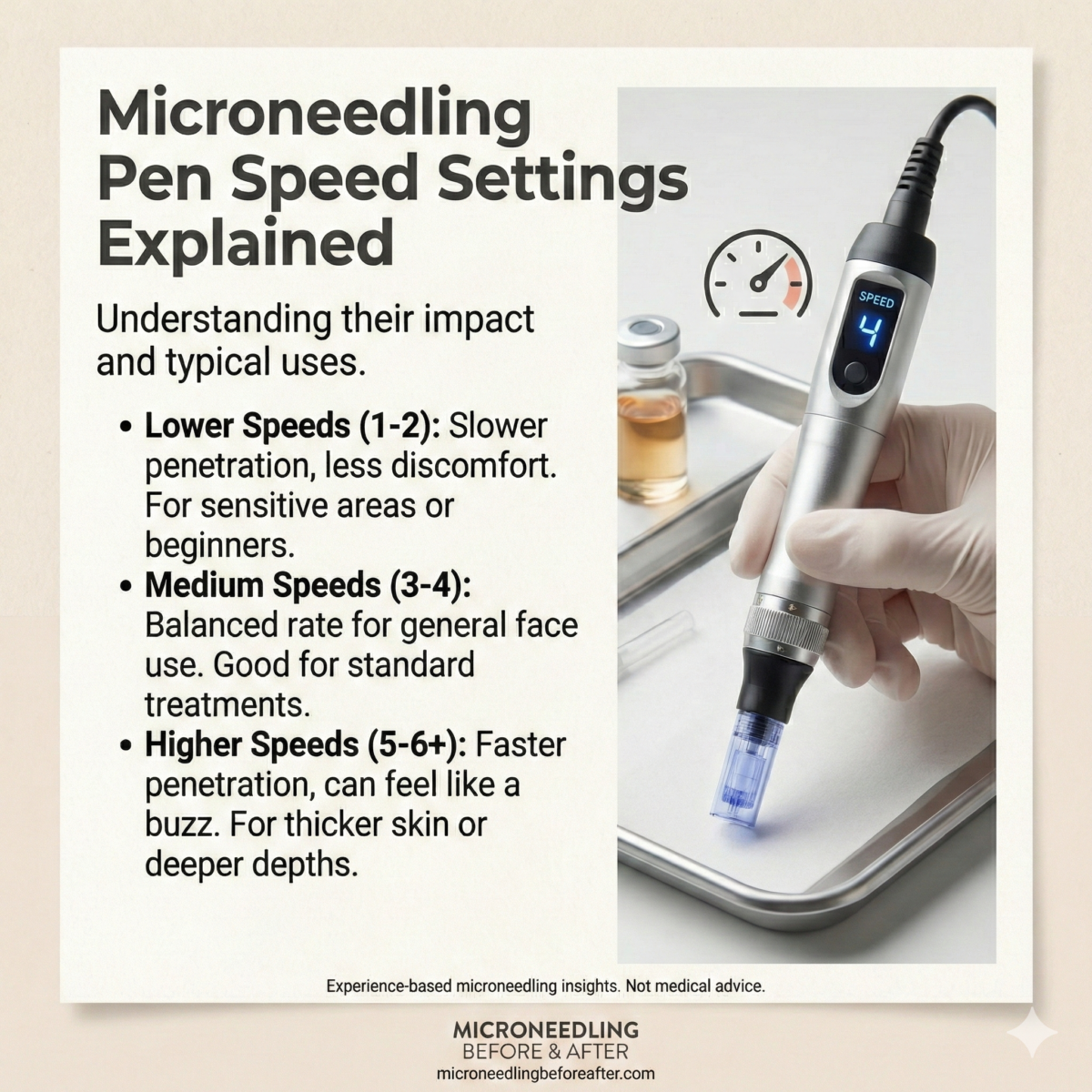
Velocidad del motor y frecuencia de carrera
Explico que la velocidad del motor se expresa de forma diferente según el fabricante: en pulsaciones por minuto, punciones por segundo o RPM. Para uso clínico, me centro en las punciones por segundo y las pulsaciones por minuto como las métricas más útiles.
Subrayo que las altas velocidades aumentan el número de punciones pero también pueden aumentar el calor por fricción y la incomodidad del paciente; por el contrario, las bajas velocidades reducen el trauma pero alargan el tiempo del procedimiento.
Explicación de los ajustes de velocidad: rangos y efectos típicos
Describo categorías generales de velocidad (baja, media, alta), proporciono una tabla práctica que asigna rangos de velocidad a indicaciones clínicas y explico cómo interpretar estas configuraciones en la práctica.
Incluyo una tabla basada en evidencia para ayudar a seleccionar velocidades según la profundidad de la aguja, el área anatómica y la intención del tratamiento.
| Categoría de velocidad | Configuración representativa* | Aproximadamente pinchazos por segundo | Rango típico de profundidad de la aguja (mm) | Usos clínicos | Ventajas | Contras |
|---|---|---|---|---|---|---|
| Bajo | 1–3 | 20–60 | 0,25–2,5 (tratamientos más profundos) | Remodelación de cicatrices profundas, estrías, tejido más grueso (parte posterior del cuello) | Entrada más controlada, menos desgarro, mejor para mayores profundidades. | Tiempo de sesión más largo, mayor fatiga del operador |
| Medio | 4–6 | 60–120 | 0,25–2,0 | Rostro general, cuello, cicatrices moderadas, PRP combinado | Equilibrio entre velocidad y control, cobertura eficiente | Molestias moderadas, se requiere una técnica cuidadosa |
| Alto | 7–12 | 120–200+ | 0,25–1,5 (tratamientos más superficiales) | Rejuvenecimiento superficial, periocular (muy superficial), sesiones rápidas | Cobertura rápida, menos tiempo en la clínica. | Mayor fricción, posible microdesgarro si la profundidad es demasiado grande |
*Los valores de configuración representativos varían según el fabricante y el modelo. Recomiendo consultar los manuales específicos del dispositivo para conocer las unidades exactas.
Señalo que los fabricantes pueden etiquetar las configuraciones numéricamente; esos números no están estandarizados. Por lo tanto, los traduzco cualitativamente para su uso práctico.
Interpretación de pinchazos por segundo
Explico que las punciones por segundo equivalen al número de agujas × golpes por segundo. Por ejemplo, un cartucho de 12 agujas a 100 golpes por segundo produce 1200 punciones por segundo en toda la matriz, pero el número de punciones por punto de piel individual depende de la frecuencia de pasada.
Subrayo que más punciones por unidad de tiempo pueden aumentar la señalización biológica pero también aumentar la carga inflamatoria transitoria.
Cómo interactúa la velocidad con la profundidad de la aguja y el tipo de tejido
Explico cómo ajustar la velocidad según la profundidad y la zona anatómica. Incluyo una segunda tabla con las combinaciones recomendadas de velocidad y profundidad como punto de partida.
| Área / Preocupación | Profundidad típica de la aguja (mm) | Categoría de velocidad recomendada | Razón fundamental |
|---|---|---|---|
| Periorbitario (debajo del ojo) | 0,2–0,5 | Bajo a medio | La piel fina requiere poca profundidad y velocidad cuidadosa para evitar hematomas y moretones. |
| Rejuvenecimiento facial completo | 0,5–1,5 | Medio | Equilibrio entre cobertura y comodidad |
| Cicatrices de acné (rodantes/en forma de vagón de carga) | 1,5–2,5 | Bajo a medio | Profundidades más profundas para la remodelación dérmica; las velocidades más lentas reducen el desgarro |
| Estrías / cuerpo | 1,5–3,0 | Bajo | El tejido grueso requiere una penetración más profunda y un control de pase cuidadoso. |
| Cuero cabelludo para el crecimiento del cabello | 0,5–2,0 | Bajo a medio | La profundidad de la aguja varía según la profundidad del folículo; velocidades más lentas para una entrada más profunda en el cuero cabelludo. |
Advierto que estos son puntos de partida y deben individualizarse en función de la comodidad del paciente, el grosor de la piel y cualquier tratamiento previo.
Por qué los tratamientos más profundos favorecen velocidades más bajas
Explico que, a mayor profundidad, la aguja penetra más tejido dérmico fibroso, lo que resiste la penetración. Las velocidades más bajas reducen el cizallamiento lateral y el efecto pistón, que puede desgarrar el tejido en lugar de crear microcanales limpios.
Agrego que las velocidades más lentas en profundidad también permiten un mejor control de la alineación de la aguja y disminuyen el riesgo de sangrado y tiempo de inactividad prolongado.
Selección práctica de la velocidad durante una sesión
Describo un marco paso a paso que utilizo: evaluación, área de prueba, titulación progresiva y documentación.
Ofrezco consejos específicos para médicos y usuarios domésticos responsables.
Parche de evaluación y prueba
Siempre evalúo el grosor de la piel, el tipo de cicatriz, la vascularidad y la tolerancia al dolor antes de seleccionar la velocidad. Luego, realizo una pequeña prueba a la profundidad planificada y a una velocidad media para observar la respuesta del tejido.
Recomiendo revisar si hay sangrado localizado, eritema excesivo o hematomas. Según la respuesta, ajusto la velocidad.
Titulación progresiva a través de zonas
Explico que a menudo uso velocidades variables dentro de una sola sesión: velocidades más lentas para mejillas con cicatrices profundas, velocidades medias para la frente y velocidades más rápidas para problemas de textura superficiales.
Hago hincapié en la documentación de las configuraciones para la reproducibilidad y las comparaciones de seguimiento.
Comunicación con el paciente y control del dolor
Recomiendo explicar a los pacientes las sensaciones que pueden esperar y usar la anestesia tópica de forma adecuada al tratar zonas más profundas. Recomiendo velocidades más lentas si el paciente refiere molestias excesivas.
También describo técnicas para reducir el dolor: presión constante, ráfagas cortas en lugar de pases continuos a alta velocidad y adormecimiento adecuado cuando esté indicado.
Combinando la microaguja con terapias complementarias
Analizo cómo la velocidad influye en el uso sinérgico de PRP, péptidos tópicos, vitamina C y ácido tranexámico, y brindo orientación sobre seguridad y momento oportuno.
Observo que los complementos cambian el perfil de riesgo y, por lo tanto, aceleran las consideraciones.
PRP y factores de crecimiento
Explico que el PRP aplicado inmediatamente después de la punción se beneficia de los microcanales abiertos, pero los tratamientos de alta velocidad podrían generar más sangrado que diluya el PRP en la superficie.
Recomiendo velocidades moderadas cuando se combina con PRP a mayor profundidad para equilibrar la formación de canales y la retención de PRP en la interfaz dérmica.
Activos tópicos y sueros
Advierto que las agujas permiten una mayor penetración de los principios activos y que algunos ingredientes (por ejemplo, retinoides, ácidos) pueden irritar el tejido subepidérmico si se aplican inmediatamente después de la punción.
Sugiero utilizar sueros estériles y equilibrados específicamente formulados para uso posterior a la punción y ajustar la velocidad para evitar una absorción sistémica excesiva o irritación.
Seguridad, contraindicaciones y control de infecciones
Proporciono orientación detallada sobre seguridad y contraindicaciones y enfatizo que la velocidad influye en el traumatismo tisular y el riesgo de infección.
Incluyo controles de procedimiento específicos que implemento en la práctica para minimizar las complicaciones.
Contraindicaciones comunes
Enumero contraindicaciones absolutas y relativas: infección activa (HSV, bacteriana), uso de isotretinoína en los últimos 6 a 12 meses, quistes de acné activos, diabetes no controlada, anticoagulación o trastornos hemorrágicos, tendencia a queloides, embarazo en algunas prácticas y expectativas poco realistas.
Aconsejo posponer el tratamiento o seleccionar profundidades superficiales y velocidades más lentas en casos límite, pero a menudo la máxima precaución es evitar la punción hasta que se resuelvan las contraindicaciones.
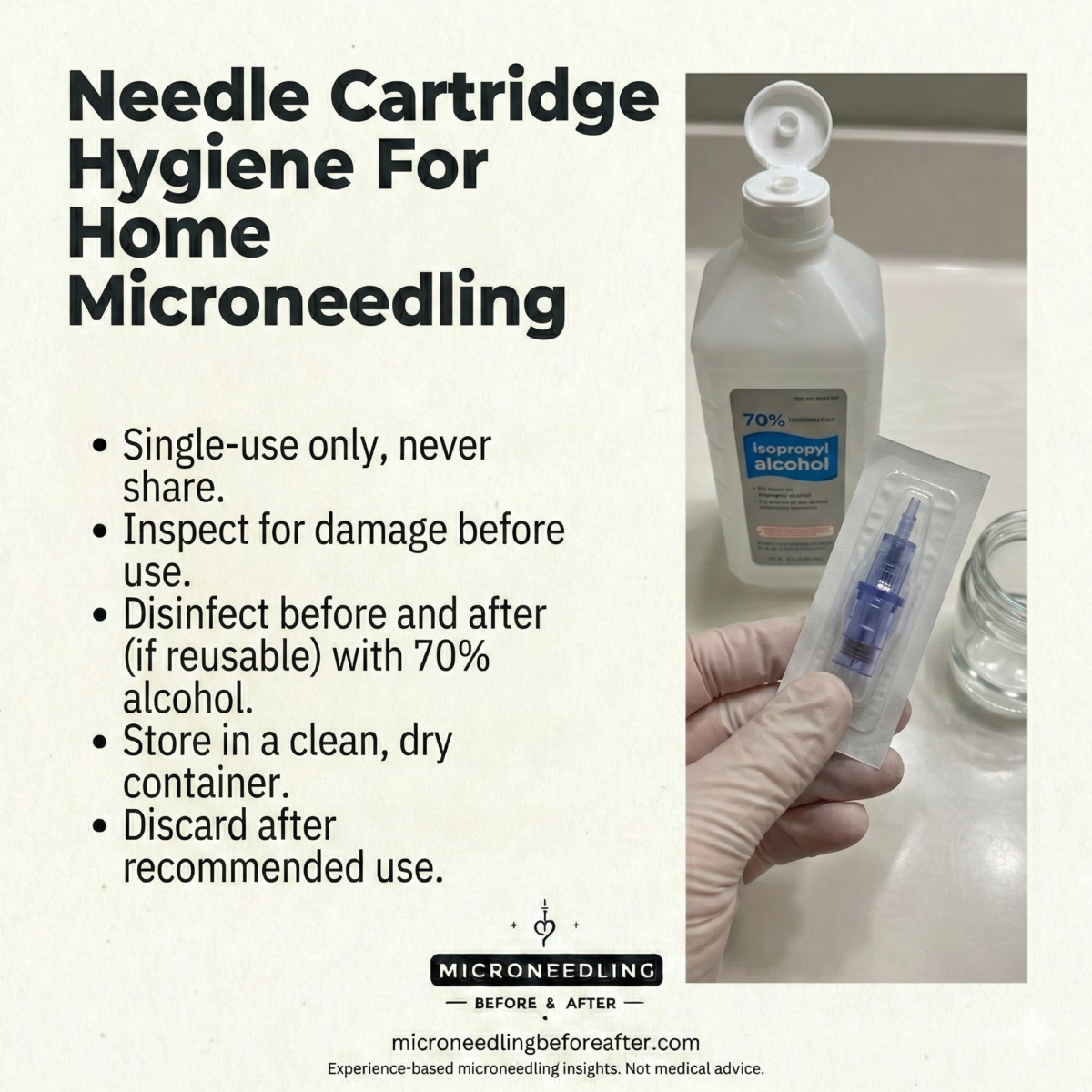
Prevención de la esterilidad y la contaminación cruzada
Describo los cartuchos estériles de un solo uso, la antisepsia cutánea (p. ej., con clorhexidina o alcohol) y el uso adecuado de guantes. Explico que las velocidades más altas pueden aerosolizar ligeramente más los líquidos, por lo que las buenas precauciones de barrera y la pulverización mínima de sueros reducen el riesgo de contaminación.
También desaconsejo pinchar con cosméticos no esterilizados y recomiendo un entorno limpio y una eliminación adecuada.
Manejo de eventos adversos
Describo los efectos secundarios comunes (eritema transitorio, edema, sangrado localizado, hematomas y formación mínima de costras) y las complicaciones más graves, como infección, hiperpigmentación y cicatrices.
Explico que ajustar la velocidad hacia abajo en sesiones posteriores a menudo ayuda a reducir el trauma repetitivo y permite la recuperación del tejido.
Cronograma de cuidados posteriores y recuperación
Ofrezco un protocolo práctico de cuidados posteriores y un cronograma esperado para obtener resultados visibles, enfatizando cómo la velocidad y la profundidad influyen en la recuperación.
Presento una tabla concisa que resume las reacciones inmediatas y tardías esperadas.
| Periodo de tiempo | Reacciones típicas | Recomendaciones de cuidado |
|---|---|---|
| Inmediatamente (0–24 horas) | Eritema, hinchazón leve, sangrado localizado | Compresas frías, limpiador suave, sin maquillaje, sueros estériles si está indicado. |
| 24–72 horas | Descamación, enrojecimiento persistente en tratamientos más profundos. | Oclusivos hidratantes, protector solar, evitar exfoliantes y ácidos activos. |
| 3–7 días | Mejora la textura de la piel, enrojecimiento residual. | Reanude el cuidado suave de la piel y controle las infecciones. |
| 2 a 12 semanas | Comienza la remodelación del colágeno, mejora visible. | Mantener el protector solar, considerar sesiones de mantenimiento |
Hago hincapié en que los tratamientos superficiales de mayor velocidad a menudo tienen una normalización más rápida, mientras que los tratamientos más profundos de baja velocidad tienen un enrojecimiento más prolongado pero una remodelación potencialmente mayor a largo plazo.
Frecuencia de tratamientos y mantenimiento
Recomiendo una serie de 3 a 6 sesiones espaciadas entre 4 y 8 semanas para la mayoría de las indicaciones, con el intervalo ajustado según la profundidad del tratamiento y la recuperación del paciente.
Recomiendo sesiones de mantenimiento cada 6 a 12 meses después de la serie inicial, dependiendo de los objetivos y la respuesta de la piel.
Evidencia clínica y estudios
Resumo la base de evidencia que respalda la eficacia de la microaguja para las cicatrices, el fotoenvejecimiento, el melasma y la caída del cabello, señalando cómo los parámetros del tratamiento influyen en los resultados.
Quiero enfatizar que si bien existen muchos estudios, los protocolos son heterogéneos y la velocidad a menudo no se informa correctamente.
Eficacia para cicatrices y fotoenvejecimiento
Tomo nota de estudios aleatorios y observacionales que demuestran una mejora en las cicatrices del acné y en la textura de la piel con microagujas, particularmente cuando se combina con PRP o factores de crecimiento tópicos.
Señalo que los protocolos que utilizan longitudes de aguja más profundas y velocidades más bajas controladas para la remodelación de cicatrices tienden a mostrar mejoras dérmicas más sólidas.
Melasma y trastornos pigmentarios
Explico que la microaguja puede mejorar la administración del agente despigmentante y mejorar las afecciones pigmentarias persistentes. Sin embargo, es necesario seleccionar cuidadosamente los parámetros, ya que un traumatismo excesivo puede exacerbar la hiperpigmentación postinflamatoria (HPI).
Recomiendo velocidades más bajas con profundidades superficiales para pacientes propensos a PIH y un uso prudente de agentes despigmentantes complementarios.
Solución de problemas comunes
Ofrezco soluciones prácticas a desafíos comunes: penetración inconsistente, sangrado excesivo, hiperpigmentación y dolor del paciente.
Cada problema incluye un protocolo para ajustar la velocidad y otros parámetros.
Penetración inconsistente o “saltos”
Si noto que los cartuchos saltan sobre la piel, primero reviso la tensión del tejido y la posición de la mano. Aumentar la tensión del tejido y reducir la velocidad suele corregir el problema.
También considero el desgaste del cartucho o las agujas desafiladas como causas y reemplazo los cartuchos en consecuencia.
Sangrado excesivo o hematomas
Reduzco la profundidad y la velocidad de la aguja en las pasadas posteriores, aplico presión para detener el sangrado y considero la interrupción temporal de los anticoagulantes en coordinación con el médico del paciente.
Evalúo si hay trastornos hemorrágicos subyacentes si el sangrado es desproporcionado.
Hiperpigmentación postinflamatoria (HPI)
Disminuyo la velocidad, reduzco la profundidad e incorporo aclaradores tópicos antes y después del tratamiento según corresponda. También recomiendo fotoprotección estricta.
Monitoreo la respuesta de la piel y pospongo las sesiones posteriores hasta que el pigmento se estabilice.
Consejos prácticos para médicos y usuarios domésticos
Describo mis reglas generales para una práctica segura y eficaz, incluida la documentación y la educación del paciente.
Incluyo listas de verificación y protocolos breves para escenarios comunes.
Lista de verificación del médico antes del tratamiento
- Realizar una historia clínica completa y una evaluación de la piel.
- Determinar la profundidad de la aguja y el plan de velocidad por zona.
- Realizar el parche de prueba y documentar la respuesta.
- Utilice cartuchos estériles de un solo uso y una antisepsia adecuada.
- Proporcionar al paciente instrucciones escritas sobre cuidados posteriores.
Hago hincapié en la importancia de documentar la velocidad, la profundidad, los pases y los complementos utilizados para la reproducibilidad.
Consideraciones sobre los dispositivos de uso doméstico
Advierto que los dispositivos domésticos suelen utilizar agujas más cortas (≤0,3–0,5 mm) y velocidades más bajas, y que los usuarios deben seguir las instrucciones del fabricante.
Recomiendo que los usuarios domésticos eviten los dispositivos de alta velocidad con agujas largas y consulten a un profesional para tratamientos más profundos.
Consideraciones éticas y regulatorias
Analizo cuestiones relacionadas con las licencias y el alcance de la práctica, y la necesidad de seguir las instrucciones del fabricante y las reglamentaciones locales.
Dejo claro que los ajustes de velocidad son un parámetro clínico que debe ser regido mediante entrenamiento y supervisión.
Formación y competencia
Exijo capacitación formal para cualquier médico que realice microagujas y recomiendo práctica supervisada para varios procedimientos antes de la práctica independiente.
Creo que es esencial comprender la mecánica del dispositivo, la esterilidad, las interacciones velocidad-profundidad y el manejo de complicaciones.
Consentimiento informado
Siempre obtengo el consentimiento informado que incluye una discusión sobre el papel de la velocidad y la profundidad, los resultados esperados, las alternativas, incluidos los regímenes solo tópicos, y los riesgos.
Documento la discusión y el plan de parámetros acordado.
Conclusión
He explicado los ajustes de velocidad de los bolígrafos de microagujas en el contexto de la mecánica del dispositivo, la justificación biológica, la selección clínica, la seguridad y la evidencia. Enfaticé que la velocidad no es una variable aislada; debe elegirse en conjunto con la profundidad de la aguja, el tipo de cartucho, las características del tejido y las terapias complementarias.
Recomiendo que los profesionales clínicos individualicen los entornos mediante parches de prueba y titulación progresiva, documenten todo y prioricen la seguridad del paciente. Los pacientes deben comprender por qué la microaguja puede funcionar cuando el cuidado de la piel por sí solo falla y buscar profesionales cualificados para tratamientos más profundos o agresivos.
Si lo desea, puedo proporcionarle una tabla de referencia rápida imprimible con recomendaciones de profundidad de velocidad adaptadas a un modelo de dispositivo específico o una plantilla de documentación y consentimiento informado de muestra que incluye configuraciones de velocidad.