¿Es posible realizar microagujas de forma segura en piel que ya tiene una barrera comprometida y, de ser así, bajo qué condiciones y precauciones?
Microagujas en la barrera cutánea comprometida
Explicaré las intersecciones entre los procedimientos de microagujas y una barrera cutánea deteriorada, y brindaré una guía práctica y basada en la evidencia para la evaluación, la planificación del tratamiento y el cuidado posterior. Mi objetivo es brindar a profesionales clínicos, estéticos y pacientes informados un marco integral para la toma de decisiones seguras y la gestión de riesgos.
Por qué es importante este tema
Reconozco que la microaguja se usa ampliamente para mejorar la textura, las cicatrices y el tono, pero crea microlesiones controladas intencionalmente. Cuando la barrera cutánea se deteriora, estos microcanales alteran los perfiles de riesgo de infección, inflamación prolongada y desestabilización de la barrera. Por lo tanto, priorizaré la seguridad, los criterios claros de contraindicación y las estrategias para rehabilitar la barrera antes de cualquier intervención invasiva.
Fundamentos del estrato córneo y la función barrera
Revisaré brevemente la fisiología cutánea relevante para que las recomendaciones posteriores se basen en el mecanismo. El estrato córneo, los lípidos y los corneocitos proporcionan una barrera dinámica que controla la pérdida de agua transepidérmica, la defensa microbiana y la penetración química.
Las tres funciones principales que destaco son: prevenir la pérdida de agua, bloquear la entrada de patógenos y regular la penetración de agentes tópicos. Cuando estas funciones se ven comprometidas, se altera tanto el entorno inmunitario local como la capacidad de reparación de la piel, lo que afecta la respuesta a la microaguja.
¿Qué quiero decir con “barrera comprometida”?”
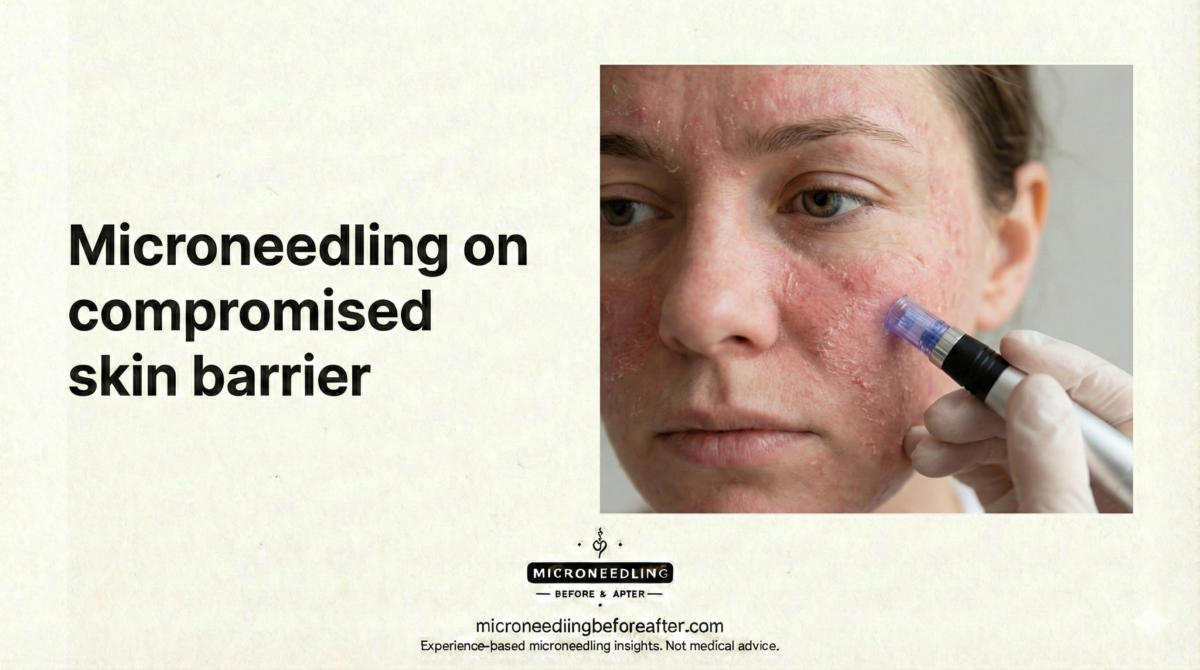
Utilizo el término “barrera comprometida” para describir la piel con integridad estructural reducida o fisiología alterada, lo que resulta en un aumento de la permeabilidad y una mayor sensibilidad. Las presentaciones clínicas típicas incluyen sequedad con fisuras, eritema y escozor, infecciones recurrentes e inflamación crónica.
Una barrera dañada puede ser transitoria (pospeeling químico, dermatitis aguda) o crónica (dermatitis atópica, daño prolongado por corticosteroides). La duración y la causa influyen en si la microaguja se pospone, modifica o considera tras la restauración.
Cómo funciona la microaguja y por qué es importante el estado de la barrera
Resumiré el mecanismo de la microaguja y lo relacionaré con sus implicaciones como barrera. Las microagujas crean microcanales que estimulan cascadas controladas de cicatrización, incluyendo la activación plaquetaria, la liberación de citocinas, el reclutamiento de fibroblastos y la remodelación del colágeno.
Estos mismos microcanales también eluden la barrera externa, lo que aumenta drásticamente la penetración tópica y permite la entrada de microbios si no se mantiene la esterilidad. En pieles con problemas de piel, la respuesta inflamatoria puede ser exagerada o desregulada, lo que aumenta la posibilidad de consecuencias adversas como inflamación persistente, infección, hiperpigmentación postinflamatoria y retraso en la cicatrización.
Profundidad, tipo de dispositivo y respuesta biológica
Explico que la longitud de la aguja, el tipo de dispositivo (rodillo, sello, bolígrafo) y la intensidad del tratamiento determinan la profundidad y la cantidad de tejido afectado. Considero que las agujas más cortas (<0,5 mm) en su mayoría epidérmicos y agujas más largas (>1,5 mm) dérmico.
En pieles con problemas, incluso las agujas más cortas pueden provocar una reactividad no deseada, ya que la epidermis puede estar ya fina o inflamada. Recomiendo adaptar la técnica a la profundidad mínima efectiva y considerar alternativas no invasivas mientras la barrera se recupera.
Causas comunes de una barrera cutánea comprometida
Enumeraré y describiré los factores contribuyentes comunes para que los lectores puedan identificar situaciones relevantes. Los factores incluyen dermatosis inflamatorias, daño iatrogénico, agresiones ambientales, uso excesivo de productos decapantes e infecciones.
- La dermatitis atópica y las afecciones eccematosas crónicas reducen el contenido lipídico y alteran la señalización inmunitaria.
- El uso excesivo de exfoliantes (AHA, BHA, retinoides, exfoliantes físicos) puede provocar la destrucción de la barrera cutánea.
- El uso indebido de corticosteroides tópicos puede adelgazar la epidermis y perjudicar los mecanismos de reparación.
- Los procedimientos faciales recientes (peelings químicos, láser, microdermoabrasión agresiva) dejan la barrera extremadamente vulnerable.
- La infección activa (herpes simple, impétigo) representa una clara contraindicación hasta que se resuelva.
Signos y síntomas que indican compromiso de la barrera
Quiero que puedas reconocer los indicadores de la consulta. Busca escozor o ardor al aplicar productos benignos, sequedad persistente con descamación, eritema mayor al basal, fisuras visibles, infecciones recurrentes e hiperreactividad a los ingredientes tópicos. Medidas objetivas como la pérdida de agua transepidérmica (TEWL) y la corneometría respaldan la evaluación cuando estén disponibles.
Si detecto signos agrupados, especialmente infección activa o atrofia inducida por corticoides, categorizaré la barrera como comprometida y evitaré la punción hasta la recuperación.
Riesgos de realizar microagujas en pieles comprometidas
Describiré los principales riesgos y explicaré sus mecanismos. Entre ellos se incluyen infección, inflamación prolongada, cicatrización, cambios pigmentarios, sensibilidad crónica y exacerbación de dermatosis subyacentes.
Infección: Los microcanales evitan las capas protectoras, lo que permite que los patógenos colonicen la dermis si la asepsia es imperfecta o el microbioma residente está desequilibrado.
Inflamación y cicatrización: una respuesta exagerada o desregulada de la herida en la piel comprometida puede provocar cicatrices hipertróficas o una curación tardía.
Pigmentación: La hiperpigmentación postinflamatoria (HPI) es más probable cuando hay inflamación basal, en particular en los tipos de piel con un índice Fitzpatrick superior.
Exacerbación: Afecciones como la rosácea o el eczema pueden reaparecer después del procedimiento, prolongando la recuperación y potencialmente causando la necesidad de esteroides sistémicos.
Contraindicaciones relativas versus absolutas
Clasifico las contraindicaciones en absolutas (condiciones que deberían excluir el procedimiento) y relativas (condiciones que requieren modificación o aplazamiento). Las contraindicaciones absolutas incluyen infección activa (VHS, bacteriana), brotes de enfermedades autoinmunes no controlados, uso activo de isotretinoína dentro del intervalo de seguridad recomendado y procedimientos ablativos recientes. Las contraindicaciones relativas incluyen eccema leve en remisión, uso reciente de esteroides tópicos sin atrofia y antecedentes de mala cicatrización de heridas o tendencia a queloides; estas requieren una evaluación individualizada.
Evaluación previa al procedimiento y toma de decisiones
Proporcionaré una lista de verificación que utilizo antes de ofrecer microagujas. Es fundamental obtener una historia clínica completa, un examen de la piel y una evaluación de riesgos y beneficios. Evalúo el historial médico (dermatitis atópica, rosácea), los procedimientos recientes, la medicación tópica/sistémica actual, los signos de infección y las expectativas del paciente.
Documento el estado basal de la piel, incluyendo eritema, descamación, fisuras y cualquier zona de atrofia. En caso de duda, pospongo el procedimiento o realizo una prueba conservadora en la zona para observar la reactividad inmediata.
Lista de verificación de evaluación práctica (tabla)
Incluyo una tabla para aclarar los elementos que reviso rutinariamente antes de realizar la microaguja.
| Elemento de evaluación | Lo que busco | Acción si es positiva |
|---|---|---|
| Infección activa (VHS, impétigo) | Vesículas, costras, dolor. | Aplazar hasta que se resuelva; antivirales/antibióticos según esté indicado |
| Uso reciente de isotretinoína | Isotretinoína en un plazo de 6 a 12 meses | Aplazar según las pautas de práctica |
| Atrofia relacionada con esteroides tópicos | Adelgazamiento, telangiectasias, facilidad para hematomas | Aplazar o limitar a terapias no invasivas; derivar |
| Actividad de eczema/psoriasis | Eritema, descamación, prurito | Tratar y estabilizar antes de la punción. |
| Renovación agresiva reciente | Descamación de la piel, heridas abiertas | Aplazar hasta la reepitelización completa |
| Tipo de piel del paciente | Clasificación de Fitzpatrick e historia de la HIP | Modificar la profundidad; asesorar sobre el riesgo de PIH |
| Expectativas y adherencia del paciente | Comprensión del tiempo de inactividad y cuidados posteriores | Educar; obtener el consentimiento |
Estrategias clínicas al considerar la microaguja en piel marginalmente comprometida
Describiré cómo modifico las técnicas cuando la barrera está ligeramente deteriorada y la microaguja sigue siendo adecuada. Mis objetivos son minimizar el trauma, reducir el riesgo de infección y favorecer la rápida restauración de la barrera.
- Reducir la profundidad de la aguja y la intensidad de la sesión; preferir 0,25–0,5 mm para la estimulación epidérmica.
- Ampliar los intervalos entre sesiones para permitir una recuperación más completa.
- Utilice cartuchos estériles de un solo uso y una técnica aséptica estricta.
- Evite realizar rejuvenecimiento químico o físico complementario en la misma sesión.
- Considere agentes tópicos que promuevan la reparación de la barrera, pero evite aquellos que sean altamente permeables y potencialmente irritantes.
Cuándo utilizar anestésicos tópicos y sueros
Soy cauteloso con los anestésicos tópicos, ya que pueden contener alcohol o conservantes que alteran aún más la barrera protectora. Si es necesario usar anestésicos tópicos, utilizo fórmulas sin conservantes y minimizo el tiempo de contacto. Para los sérums, selecciono fórmulas estériles y sencillas (factores de crecimiento o ácido hialurónico en vehículos estériles y sin conservantes) solo si la piel no presenta signos de infección activa.
Cuidados posteriores: rehabilitación de la barrera
Doy mucha importancia al cuidado posterior para la reparación de la barrera cutánea. Mis prioridades son: mantener la hidratación, prevenir infecciones, minimizar la inflamación y evitar irritantes. Indico a mis pacientes que utilicen limpiadores suaves sin perfume, emolientes oclusivos y protector solar.
Recomiendo aplicar un humectante (p. ej., ácido hialurónico) en capas con un oclusivo (p. ej., vaselina o ungüento rico en ceramidas) para retener la hidratación. El protector solar es esencial, ya que la exposición a los rayos UV puede empeorar la hiperpigmentación cutánea y retrasar la reparación epidérmica.
Detailed aftercare timeline
I provide a practical timeline I favor for uncomplicated sessions that can be adjusted for compromised skin.
- Immediate (0–12 hours): Gentle cleansing with sterile saline or mild cleanser; apply sterile, preservative-free hyaluronic serum if tolerated, then occlusive emollient. Avoid makeup.
- 12–72 hours: Continue gentle cleansing twice daily, frequent emollients, avoid active ingredients (retinoids, acids), use physical or mineral SPF once re-epithelialized.
- Day 3–14: Gradual reintroduction of barrier-supportive actives (niacinamide, ceramides); return to stronger actives only after full barrier recovery and clinician clearance.
- Ongoing: Emphasize regular barrier-supportive skincare and sun protection.
Ingredients and product selection: what I use and avoid
I find a clear list of preferred and contraindicated ingredients helps reduce confusion. I emphasize sterile, non-irritating, barrier-repairing formulations and avoidance of alcohol, fragrances, high-concentration acids, and potentially photosensitizing agents.
Recommended versus avoid list (table)
This table summarizes common ingredients I recommend or avoid in the immediate peri-procedure window.
| Use immediately post-procedure | Avoid immediately post-procedure |
|---|---|
| Sterile hyaluronic acid (low molecular weight) | Alcohol-containing toners |
| Petrolatum or occlusive ointments | High-strength AHAs/BHAs |
| Ceramide-rich creams | Retinoids (for 7–14 days) |
| Niacinamide (after 48–72 hours if tolerated) | Benzoyl peroxide (irritating) |
| Gentle, fragrance-free cleansers | Exfoliating scrubs |
| Mineral sunscreen once re-epithelialized | Chemical sunscreens with potential irritation if skin reactive |
Device choices: professional clinic versus at-home units
I always advise caution with at-home microneedling devices, particularly on compromised skin. Professional devices deliver controlled depths, disposable sterile cartridges, and are applied in an aseptic environment by trained practitioners.
At-home rollers often lack consistent depth control, may be reused between sessions, and increase the risk of microtrauma and infection. If patients insist on at-home maintenance, I recommend short needles (<0.25 mm), rigorous hygiene, and only after full barrier recovery.< />>
Comparative table: clinic-based vs at-home microneedling
I provide a simple comparison to clarify differences and safety considerations.
| Característica | Clinic-based microneedling | At-home microneedling |
|---|---|---|
| Control de profundidad de la aguja | Precise, adjustable | Often fixed, inconsistent |
| Esterilidad | Single-use sterile cartridges, trained aseptic technique | Reusable devices, variable cleaning |
| Clinical assessment | Pre-procedure evaluation possible | Self-assessment only |
| Ability to treat complications | Immediate professional care | Delay to seek help may worsen outcomes |
| Suitability for compromised skin | Conditional with modifications | Generally discouraged |
Contraindications and special populations
I state clear contraindications and note special considerations for patients with certain systemic conditions. Absolute contraindications include active skin infection, recent isotretinoin within safe timeframe, uncontrolled autoimmune disease flares, keloid history with active scarring tendencies, and pregnancy for some devices or adjuncts.
For immunosuppressed patients or those on systemic steroids, I proceed with extreme caution or defer to alternative therapies. For darker Fitzpatrick skin types, I counsel extensively on PIH risk and consider conservative approaches or alternative modalities with lower inflammatory potential.
Pregnancy, breastfeeding, and medications
I explain that while microneedling itself is not universally contraindicated in pregnancy, adjunctive topical agents, local anesthetics, and associated procedures may be. I evaluate each case individually and often defer elective cosmetic needling during pregnancy or breastfeeding until after consultation with obstetric care.
Medications like systemic retinoids require established washout periods; I adhere to guideline-recommended intervals to reduce risks of delayed healing and scarring.
Managing complications: early detection and treatment
I outline an algorithmic approach I use for the most common complications. Prompt recognition short-circuits escalation.
- Infection: If local erythema, increasing pain, purulence, or systemic symptoms appear, I initiate culture-directed topical or systemic antibiotics and consider incision/ drainage if abscess forms.
- Herpes simplex reactivation: For known HSV-positive patients, prophylactic antivirals around the procedure reduce risk; treat active outbreaks with systemic antivirals and defer needling.
- Persistent inflammation/flare: I treat with topical anti-inflammatory strategies (low-potency steroids short-term if indicated, or non-steroidal anti-inflammatories like topical calcineurin inhibitors in some scenarios), guided by dermatology when needed.
- PIH: Early use of sun protection, topical brightening agents after re-epithelialization (azelaic acid, niacinamide), and avoid further trauma. I may refer for targeted pigment therapies if persistent.
When to refer to dermatology or specialty care
I am quick to refer if there is any uncertainty about healing, evidence of severe infection, unexpected scarring, or systemic symptoms. For complex dermatologic conditions (e.g., severe atopic dermatitis, autoimmune blistering diseases), I coordinate care and prefer dermatology-directed timing and protocols.
Rehabilitation protocols to restore barrier pre-procedure
I provide an evidence-informed plan for rehabilitating a compromised barrier before any elective microneedling. The goal is objective improvement in hydration, reduction of inflammation, and normalization of TEWL when possible.
- Short course of topical emollients rich in ceramides and cholesterol to rebuild lipid matrix.
- Reduce or pause active exfoliants and retinoids for 2–4 weeks or until tolerance returns.
- If inflammatory dermatosis is active, treat with targeted therapies (topical steroids, calcineurin inhibitors, or systemic agents as indicated) until controlled.
- Consider patch testing or supervised reintroduction of sensitizing actives when the patient is ready.
I typically wait at least 2–6 weeks of documented clinical improvement before reconsidering needling, with longer intervals for severe or chronic conditions.
Practical product and regimen example
I often recommend: twice-daily gentle cleansing, immediate post-cleanse application of a humectant serum, thick ceramide-rich cream morning and evening, and petrolatum occlusive at night for 1–2 weeks. I monitor clinical signs weekly and reassess readiness using a checklist that includes absence of active inflammation, reduced TEWL symptoms, and improved tolerance.
Evidence summary and knowledge gaps
I summarize key evidence while acknowledging limitations. Clinical studies show microneedling effectively treats scars and photoaging with generally favorable safety profiles in healthy skin. However, there is sparse high-quality evidence specifically addressing outcomes in pre-existing barrier compromise.
Randomized trials rarely enroll patients with active dermatitis or recent barrier injury; therefore, recommendations often derive from mechanism-based reasoning, expert consensus, and smaller observational studies. I therefore practice conservative modifications and prioritize individualized assessment.
Areas needing more research
I identify actionable research gaps that matter clinically. These include randomized controlled trials on microneedling safety in mild-to-moderate barrier dysfunction, optimal device settings for compromised skin, and the role of specific barrier-repair adjuncts in improving outcomes.
Practical recommendations — step-by-step summary
I provide a concise stepwise approach clinicians or informed patients can apply.
- Assess barrier status thoroughly and document findings.
- Classify risk: absolute contraindication, relative contraindication, or acceptable with modifications.
- If marginally compromised, rehabilitate barrier with emollients and anti-inflammatory therapy until improved.
- If proceeding, use conservative needle depths, sterile technique, and single-use cartridges.
- Avoid combining with other resurfacing treatments in the same session.
- Use sterile, gentle post-procedure emollients and occlusion; avoid irritants.
- Monitor closely for infection, prolonged inflammation, or pigmentary changes and act early.
- Refer to dermatology for any uncertainties or complications.
Case scenarios to illustrate application
I present brief hypothetical cases to apply principles.
Case 1: A 35-year-old with mild eczema well-controlled on emollients requests microneedling for acne scarring. I treat and stabilize the eczema for 4–6 weeks, confirm clinical remission, then proceed with shallow needle depths and extended recovery intervals.
Case 2: A 28-year-old who used topical isotretinoin two months ago presents for needling. I defer until the recommended washout period has passed (commonly 6–12 months depending on dosing and guidelines) due to impaired healing risk.
Case 3: A 45-year-old with recent chemical peel and visible peeling asks to combine treatments. I defer microneedling until complete re-epithelialization to avoid compounding barrier injury and infection risk.
Final considerations and conclusion
I prioritize patient safety and realistic outcome expectations. Microneedling can be valuable but is not without risk when the skin barrier is compromised. Clinicians should balance therapeutic intent with a conservative, evidence-informed approach that emphasizes barrier restoration, aseptic technique, individualized device settings, and close follow-up.
I recommend documenting counseling, performing a thorough pre-procedure assessment, and avoiding shortcuts with at-home devices in compromised skin. When in doubt, I prefer to delay the procedure and restore the barrier rather than treat preventable complications.
If you would like, I can provide a printable pre-procedure checklist, a tailored rehabilitation regimen based on a specific clinical scenario, or references to clinical guidelines that I use in practice.