?Have you considered how microneedling recovery will feel for your skin type and what specific steps I would take if I had oily versus dry skin?
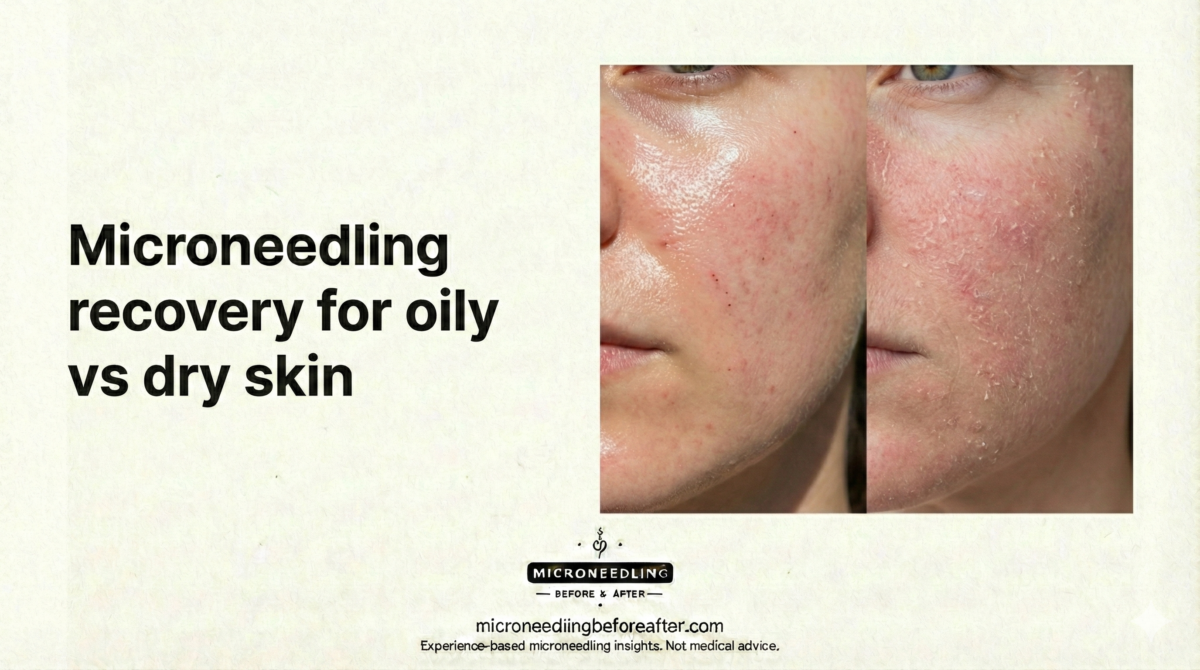
Microneedling Recovery For Oily Vs Dry Skin
I will explain the recovery process for microneedling with a focus on the differences between oily and dry skin. I draw on clinical principles and practical aftercare to offer a clear, step-by-step guide that I would follow or recommend to clients.
What microneedling does and why recovery matters
Microneedling creates controlled micro-injuries in the skin to stimulate collagen production and enhance topical product absorption. I always emphasize that recovery is not just a passive wait — it is an active period in which proper care influences outcomes such as texture improvement, scar remodeling, and pigmentation control. Recovery strategies should be tailored to skin type because barrier function, sebum production, and propensity for inflammation differ between oily and dry skin.
How skin type changes the recovery process
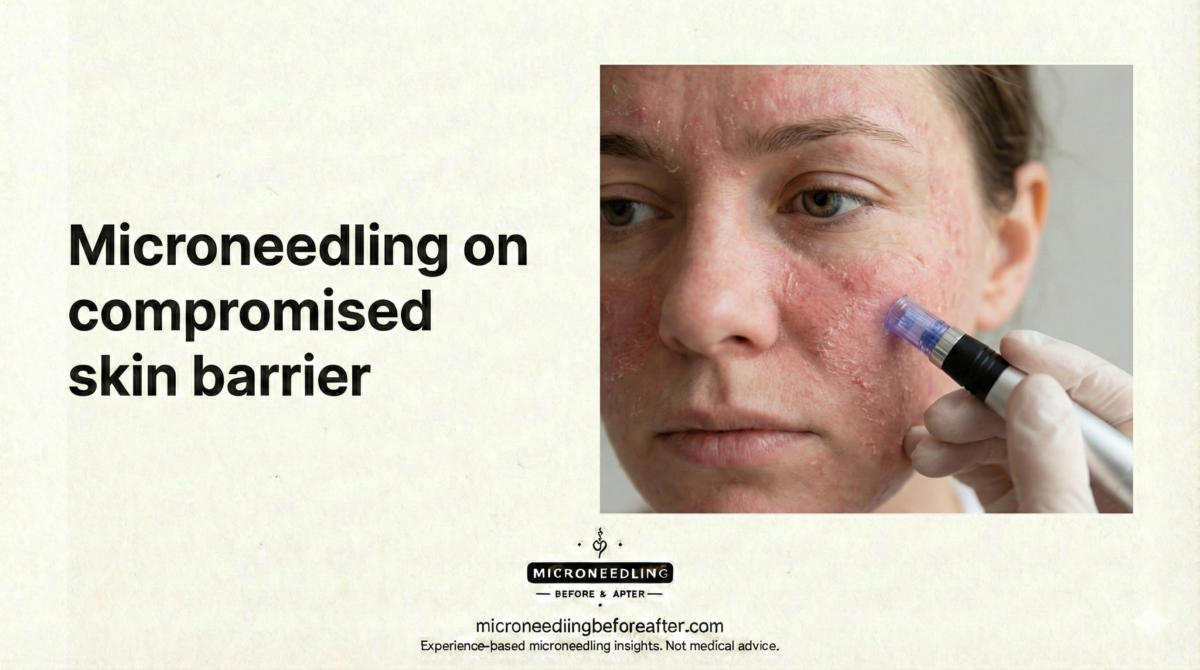
I consider skin type a primary variable when planning microneedling aftercare. Oily skin typically produces more sebum and may be more prone to congested pores or post-procedure acne flare-ups. Dry skin often has impaired barrier function, increased transepidermal water loss (TEWL), and more noticeable tightness and flakiness after treatment. Understanding these physiological differences allows me to recommend appropriate cleansers, moisturizers, and protective measures.
Key physiological differences between oily and dry skin
I want to summarize the most important functional differences so my aftercare recommendations make sense.
- Sebum production: Oily skin produces more sebum, which can trap bacteria and increase the risk of inflammation after microneedling. Dry skin has low sebum and struggles with moisture retention.
- Barrier integrity: Dry skin often has a compromised barrier that requires immediate support to reduce TEWL and irritation. Oily skin may have an intact but acne-prone barrier.
- Healing tendencies: Oily skin can appear less inflamed visually due to oil sheen but may develop comedones or pustules. Dry skin shows more pronounced flakiness, redness, and tightness.
- Pigmentation risk: Both types can develop post-inflammatory hyperpigmentation (PIH), but oily and acne-prone skin may be at slightly higher risk if inflammatory lesions occur.
Typical microneedling timeline: what to expect
I find it helpful to present a general timeline that applies to all skin types, then highlight the differences for oily and dry skin within each stage.
Immediate (0–24 hours)
I expect erythema (redness), warmth, and mild pinpoint bleeding right after the procedure. The face can feel tight or slightly puffy. For oily skin, excess sebum may appear within hours, whereas dry skin often feels uncomfortably tight.
Early (24–72 hours)
Redness and sensitivity gradually subside. For dry skin, flaking and peeling begin to show around day two or three as the skin sheds micro-damaged cells. For oily skin, I monitor for clogged pores and tiny pustules; cleansing frequency and non-comedogenic products become crucial.
Intermediate (3–7 days)
Most of the visible surface recovery occurs. Dry skin may continue to flake and feel tight for up to a week, and I recommend barrier repair protocols. Oily skin generally returns to baseline sheen but the risk of breakout persists; I advise gentle but effective cleansing and anti-microbial measures if necessary.
Long-term (weeks 2–12)
Collagen remodeling occurs over weeks to months. I monitor results such as improved texture, scar softening, and pigmentation changes. I advise sun protection and gradual introduction of active ingredients based on skin type and tolerance.
Side-by-side recovery comparison
I use this table to give a quick, practical comparison of symptoms and concerns for oily vs dry skin during recovery.
| Recovery phase | Oily skin — common issues | Dry skin — common issues |
|---|---|---|
| 0–24 hours | Excess oiliness, possible clogged pores | Intense tightness, stinging |
| 24–72 hours | Increased risk of pustules, shallow acne flares | Flaking, scaling, pronounced tightness |
| 3–7 days | Return to baseline oiliness; watch for comedones | Continued desquamation; needs barrier repair |
| 2–12 weeks | Higher PIH risk if inflammation occurs | Sensitive to actives; pigmentation risk if barrier not restored |
Pre-treatment preparation I recommend
I always prepare skin to reduce complications and improve outcomes. Preparation varies by skin type.
General pre-treatment recommendations
I usually advise stopping retinoids or strong chemical exfoliants 3–7 days before, avoiding active sun exposure, and arriving with clean skin without makeup. I assess medications and medical history to rule out contraindications like isotretinoin use in the recent past.
Specific advice for oily skin
I may recommend a course of topical benzoyl peroxide or a short-term antimicrobial skincare routine if there is active acne. I tell clients to avoid starting new, potentially irritating products in the weeks before the procedure.
Specific advice for dry skin
I focus on improving barrier function before microneedling. I often suggest intensive hydration with ceramide-rich moisturizers and temporary discontinuation of strong drying agents (benzoyl peroxide, alcohol toners). I may also recommend a gentle humectant like hyaluronic acid leading up to treatment.
Immediate aftercare (first 24–48 hours)
Immediate aftercare sets the tone for recovery. I provide clear instructions I would follow myself.
Cleansing and protection
I gently clean treated skin with a mild, non-foaming cleanser and lukewarm water. I avoid rubbing or using abrasive cloths. For both oily and dry skin, I recommend avoiding makeup for at least 24 hours and using only products approved by the practitioner.
Cooling and calming
I often recommend cool compresses (not ice directly on skin) to reduce swelling and warmth. Anti-inflammatory measures such as topical soothing serums (centella asiatica, panthenol, or azelaic acid for acne-prone skin) can help depending on tolerance.
Dressing and contamination avoidance
I stress that the treated skin is essentially a micro-wounded surface. I advise avoiding touching the face with unclean hands, staying away from sauna/steam rooms, and avoiding swimming pools for at least 48–72 hours.
Day-by-day recovery plan for oily skin
I outline a specific day-by-day protocol that I would follow for oily skin to reduce breakouts and support healing.
Day 0 (procedure day)
- Cleanse gently and follow practitioner’s immediate post-procedure topical application (often an antimicrobial or healing serum).
- Avoid makeup and sunscreen application if the practitioner advises waiting; otherwise use a mineral SPF only if allowed.
- Use cool compresses for comfort.
I emphasize that initial oils will appear and they are not inherently bad, but I avoid aggressive stripping cleansers.
Day 1–2
- Cleanse twice daily with a gentle, pH-balanced foaming cleanser to remove excess sebum while minimizing irritation.
- Apply a lightweight, non-comedogenic hydrating serum (hyaluronic acid-based) and a non-comedogenic, hydrating moisturizer.
- If acne-prone, I may use topical azelaic acid as it has anti-inflammatory and anti-microbial properties and is generally better tolerated than benzoyl peroxide immediately after microneedling. I consult my practitioner before starting any actives.
Day 3–5
- Continue gentle cleansing and light moisturizing.
- Introduce topical niacinamide (up to 5%) to help regulate sebum production and reduce inflammation if tolerated.
- Keep monitoring for signs of infection or pustular breakout; contact my practitioner if lesions become widespread or painful.
Day 6–14
- Gradually reintroduce routine anti-acne treatments (benzoyl peroxide, salicylic acid) only after confirming the skin barrier is recovering and with practitioner guidance.
- Continue strict sun protection and avoid occlusive heavy creams that can trap sebum and exacerbate comedones.
Day-by-day recovery plan for dry skin
I provide a parallel protocol for dry skin, focused on restoring the barrier and minimizing flaking.
Day 0 (procedure day)
- Use the practitioner’s recommended healing serum or ointment; often a thicker occlusive is suggested to reduce TEWL for dry skin.
- Avoid makeup for at least 24 hours and keep the skin moisturized with a healing cream as advised.
Day 1–2
- Cleanse with an ultra-gentle, non-foaming cleanser once or twice daily, using minimal water contact to reduce stinging.
- Apply hydrating serums (low molecular weight hyaluronic acid) followed by a rich ceramide- and cholesterol-containing moisturizer to rebuild lipid layers.
- Use an occlusive like petroleum jelly at night if allowed, to lock in moisture.
Day 3–5
- Expect flaking or peeling; use gentle physical exfoliation only if advised by my practitioner (rarely recommended this early).
- Continue barrier-repair focused moisturization with twice-daily application.
- Avoid hot showers, alcohol-based toners, and any drying ingredients.
Day 6–14
- Slowly reintroduce mild actives like peptide serums or low-concentration vitamin C if tolerated; avoid retinoids until the skin is fully healed.
- Continue strict sun protection and maintain a rich moisturizer routine.
Recommended ingredients and products
I find it efficient to present recommended ingredients in a table that contrasts what I recommend for each skin type and what to avoid.
| Skin type | Ingredients I recommend | Ingredients I avoid in early recovery |
|---|---|---|
| Oily | Hyaluronic acid, niacinamide, azelaic acid, low-irritant cleansers, light non-comedogenic moisturizers | Heavy occlusives, mineral oils that may clog, strong retinoids, physical exfoliants |
| Dry | Ceramides, cholesterol, fatty acids, hyaluronic acid, panthenol, occlusives (petrolatum) | Alcohol-based toners, benzoyl peroxide (immediately post), strong acids, retinoids early on |
I emphasize that specific product brands are less important than ingredient tolerability and non-comedogenic labeling for oily skin.
Antimicrobials and acne management
I may include topical azelaic acid or prescription antimicrobials for those with active acne. If I suspect bacterial overgrowth or significant infection, I consult or refer to a dermatologist for possible oral antibiotics or other interventions.
Sun protection and pigmentation prevention
I consider sun protection during recovery to be non-negotiable. UV exposure increases the risk of PIH and impairs collagen remodeling.
Sunscreen recommendations
I recommend a broad-spectrum SPF 30–50 every day. For oily skin, I prefer lightweight mineral or chemical sunscreens labeled non-comedogenic. For dry skin, I choose sunscreens with added moisturizers or combine with a hydrating layer beneath sunscreen.
Additional pigmentation control
If PIH is a concern, I consider introducing topical lightening agents like tranexamic acid, niacinamide, or low-dose hydroquinone only after full re-epithelialization and with professional guidance. I avoid starting potent bleaching agents immediately after microneedling.
When to contact a professional
I instruct clients to seek professional help if they experience any of the following:
- Increasing pain, warmth, or spreading redness beyond expected areas (possible infection).
- Purulent drainage or increasing pustular lesions.
- Severe swelling or signs of an allergic reaction (urticaria, systemic symptoms).
- New, rapidly spreading pigmentation changes.
I stress that early intervention prevents long-term complications.
Managing complications: infection, PIH, and acne flares
I will describe how I manage the most common complications and how prevention differs by skin type.
Infection
If I suspect infection, I advise stopping any potent topicals and contacting a healthcare provider. Treatment may include oral or topical antibiotics based on culture and clinical judgment.
Post-inflammatory hyperpigmentation (PIH)
PIH management includes strict sun protection and later introduction of topical lightening agents under supervision. For oily skin with acne-related PIH, controlling inflammation quickly reduces PIH risk.
Acne flares
I work with a practitioner to balance antimicrobial therapy and anti-inflammatory agents. For oily skin, early topical azelaic acid or a temporary regimen including topical antibiotics may be helpful. For dry skin that develops acne from occlusive moisturizers, I modify the skincare routine to lighter, non-comedogenic hydrators.
Returning to active ingredients
I emphasize a cautious, phased approach to reintroducing strong actives.
Retinoids
I typically wait at least 7–14 days before reintroducing retinoids, often longer for deep microneedling or dry skin. I start with lower concentrations and apply every other night, gradually building tolerance.
Chemical exfoliants (AHAs/BHAs)
I avoid acids for 5–7 days minimum and longer for sensitive or dry skin. For oily, acne-prone skin, salicylic acid can be useful but should be reintroduced slowly and at lower concentrations.
Vitamin C and other actives
Vitamin C can be irritating; I reintroduce it after re-epithelialization and monitor for stinging. Peptides and growth-factor-containing serums are generally safe earlier and can support healing.
Professional vs. at-home microneedling and recovery differences
I will clarify recovery differences depending on needle depth and setting.
In-office professional microneedling
Professional procedures typically use longer needles and create deeper microchannels, leading to more pronounced redness and a slightly longer healing phase. I follow practitioner aftercare closely and expect a stronger emphasis on avoiding contamination and strict sun protection.
At-home microneedling (derma rollers)
At-home devices use shorter needles and produce milder, more superficial effects. Recovery is generally faster and less intense, but the risk of improper sterilization and infection is higher if protocols are not followed. I recommend caution and encourage professional treatments for significant skin concerns.
Long-term maintenance and expectations
I set realistic expectations: microneedling stimulates collagen over months, and multiple sessions are often needed. I recommend a maintenance schedule and complementary treatments.
Number of sessions and intervals
I typically recommend 3–6 sessions spaced 4–6 weeks apart for collagen remodeling treatments, but individualized plans depend on skin concern, needle depth, and response. For superficial rejuvenation, fewer sessions may suffice.
Complementary treatments
I often pair microneedling with controlled topical therapies like growth-factor serums or PRP (platelet-rich plasma) under supervision. I caution against layering potent actives immediately post-procedure and recommend using supportive, hydrating serums during the healing period.
Practical lifestyle tips during recovery
I include lifestyle measures I apply myself to support optimal healing.
- Sleep: I prioritize adequate sleep to support tissue repair.
- Diet: I eat protein-rich foods and maintain sufficient hydration and micronutrients, especially vitamin C and zinc.
- Avoid smoking and excessive alcohol: Both impair wound healing and collagen production.
- Exercise: I avoid strenuous exercise that induces heavy sweating for 48–72 hours to reduce contamination risk.
Makeup and social considerations
I understand many people want to return to social activities quickly. I give practical guidance on makeup timing and camouflage options.
Makeup timeline
I usually advise avoiding makeup for at least 24 hours, but for deeper treatments waiting 48–72 hours is safer. When I reintroduce makeup, I select non-comedogenic mineral cosmetics and apply with clean tools.
Camouflage for redness
I suggest green-tinted formulations or color-correcting products only after the skin has re-epithelialized and with caution for patch testing, especially on dry skin to avoid further irritation.
Cost considerations and choosing a provider
I recommend seeking an experienced practitioner who follows sterile protocols. Lower-cost treatments may be tempting but can increase the risk of poor technique and complications. I look for providers with clear pre- and post-care instructions and a clean, professional setting.
Case examples: how I would tailor recovery plans
I present two hypothetical cases to illustrate practical differences.
Case 1: Oily, acne-prone 28-year-old
I would pre-treat active acne, consider antimicrobial stewardship, use lightweight hydrating serums and niacinamide during healing, and reintroduce salicylic acid slowly. I would monitor closely for pustules and advise rapid contact if infection is suspected.
Case 2: Dry, sensitive 45-year-old
I would strengthen the barrier before treatment, use occlusive and ceramide-rich products immediately after, avoid actives for a longer period, and reintroduce retinoids slowly over several weeks. I would manage peeling and flaking proactively.
Summary and practical checklist
I summarize the essential points and provide a concise checklist I follow.
- Pre-treatment: Stop strong actives, improve barrier for dry skin, reduce inflamed acne for oily skin.
- Immediate care: Gentle cleansing, soothing serums, avoid contamination and sun.
- First week: Oily — manage sebum with non-comedogenic hydrators; Dry — prioritize occlusion and ceramides.
- Reintroduction of actives: Gradual; longer pause for dry and for deeper procedures.
- Watch for complications: Infection, PIH, acne flares — contact a professional early.
- Maintenance: Multiple sessions may be needed; combine with sun protection and healthy lifestyle.
Checklist (printable):
| Task | Oily | Dry |
|---|---|---|
| Pre-treatment prep | Control acne; avoid new products | Build barrier; hydrate |
| Day 0–2 | Gentle cleanser twice daily; hyaluronic acid; avoid heavy creams | Gentle cleanser; rich ceramide moisturizer; occlusive at night |
| Day 3–7 | Introduce niacinamide; monitor for pustules | Continue barrier repair; avoid actives |
| Week 2+ | Gradually reintroduce actives | Slow reintroduction of actives; use peptides first |
| Sun protection | Non-comedogenic SPF daily | Hydrating SPF daily |
Final recommendations and my closing professional note
I recommend approaching microneedling with a plan tailored to your skin type. If I had to prioritize three actions during recovery they would be: protect the skin from sun, support the barrier (especially if dry), and avoid introducing strong actives too early. I also emphasize the value of working with a qualified practitioner who provides individualized aftercare instructions. If you want, I can help draft a personalized post-procedure regimen based on your exact product preferences, medical history, and the depth of microneedling you plan to undergo.