? Have you ever wondered why microneedling pens with adjustable speed settings can produce different outcomes even when the needle depth remains the same?
Microneedling Pen Speed Settings Explained
I will explain what microneedling pen speed settings mean, how they affect treatment outcomes, and why microneedling can succeed in cases where topical skincare fails. I will cover the mechanics, clinical reasoning, practical recommendations, safety considerations, and aftercare so that you can make informed decisions whether you are a clinician, technician, or an informed patient.
Introduction to microneedling pens
I use microneedling pens in clinical practice and consult with patients about device choices and protocols. These devices have become popular because they produce controlled microinjuries that stimulate collagen and improve topical product delivery.
In this section I outline the difference between pen-style devices and other forms of needling, and why adjustable speed matters. I will emphasize the relationship between speed, needle count, and tissue response.
What a microneedling pen is
I consider a microneedling pen to be a handheld, motorized device that repeatedly drives an array of tiny needles into the skin.
I explain that unlike manual rollers, pens allow control of needle depth and needling speed, provide perpendicular entry to the skin, and reduce drag and tearing. These technical differences matter for outcomes and safety.
Why adjustable speed matters
I describe how speed changes the number of micro-injuries created per second and alters mechanical shear forces, pain perception, and heat generation.
I also note that speed interacts with needle length, cartridge type, and the anatomical area treated. Optimal combinations reduce unnecessary trauma while maximizing regenerative signaling.
How microneedling works biologically
I will present the biological basis for microneedling’s effectiveness, emphasizing the wound-healing cascade and enhanced transdermal delivery.
This section aims to clarify mechanisms so that speed settings make sense in context of tissue response.
The wound-healing cascade and collagen induction
I explain that controlled micro-injuries initiate hemostasis followed by inflammation, proliferation, and remodeling. These stages recruit platelets, neutrophils, macrophages, fibroblasts, and endothelial cells.
I make the point that collagen types I and III are synthesized during remodeling, which improves skin texture, firmness, and scar remodeling over weeks to months. Speed influences the density and pattern of microinjuries and thereby the strength of signaling.
Enhanced delivery of topical agents
I describe how microchannels reduce the barrier function of the stratum corneum and allow greater penetration of serums, peptides, growth factors, and other actives.
I emphasize that microneedling is not merely a delivery method but also a biological stimulus. When topical agents are used immediately after treatment, their deeper contact can augment outcomes, provided sterilization and ingredient safety are observed.
Why microneedling works when topical skincare doesn’t
I frequently explain to patients that topical products may fail because of limited penetration, insufficient biological stimuli, or chronic tissue changes that need a wound-healing reset.
Here I break down the key reasons microneedling can succeed where skincare alone is inadequate.
Barrier limitations of topical skincare
I point out that the stratum corneum restricts the passage of many active molecules, particularly large peptides, proteins, and growth factors.
I explain that even well-formulated products may not reach the viable epidermis or dermis where target cells reside. Microneedling overcomes this barrier by physically creating channels.
Chronic damage and the need for remodeling
I discuss how photoaging, acne scarring, and long-standing laxity involve architectural changes in dermal collagen that topical antioxidants or retinoids cannot fully reverse.
I emphasize that microneedling triggers a remodeling process which replaces disorganized extracellular matrix with newer collagen and elastin, producing structural improvement rather than only biochemical modulation.
Cellular recruitment and local growth factor release
I note that microinjury recruits immune cells and platelets that release signaling molecules — transforming growth factor-beta (TGF-β), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) — which topicals alone rarely induce to the same magnitude.
I highlight that this local orchestration of repair is a core advantage of mechanical stimulation over passive topical application.
Microneedling pen components and parameters
I explain the main device parameters that influence outcomes: needle depth, needle count/arrangement, needle material, cartridge design, and speed.
Under each parameter I provide practical considerations so you understand how speed fits into the overall protocol.
Needle depth and tissue targeting
I explain that depth is the primary determinant of which skin layers are engaged — superficial epidermis, papillary dermis, or reticular dermis.
I also advise that deeper penetration generally requires slower, more controlled movements and often lower speeds to avoid unnecessary tearing and pain.
Needle cartridge design, count, and configuration
I describe how cartridges vary in the number and arrangement of needles, which changes the area treated per pass and the pressure distribution on the skin.
I mention that higher needle counts can reduce the number of passes required, but speed must be adjusted to ensure consistent entry and minimal shearing.
Motor speed and stroke frequency
I explain that motor speed is reported differently by manufacturers: as strokes per minute, punctures per second, or RPM. For clinical use I focus on punctures per second and strokes per minute as the most useful metrics.
I stress that high speeds increase the number of punctures but can also increase frictional heat and patient discomfort; conversely, low speeds reduce trauma but lengthen procedure time.
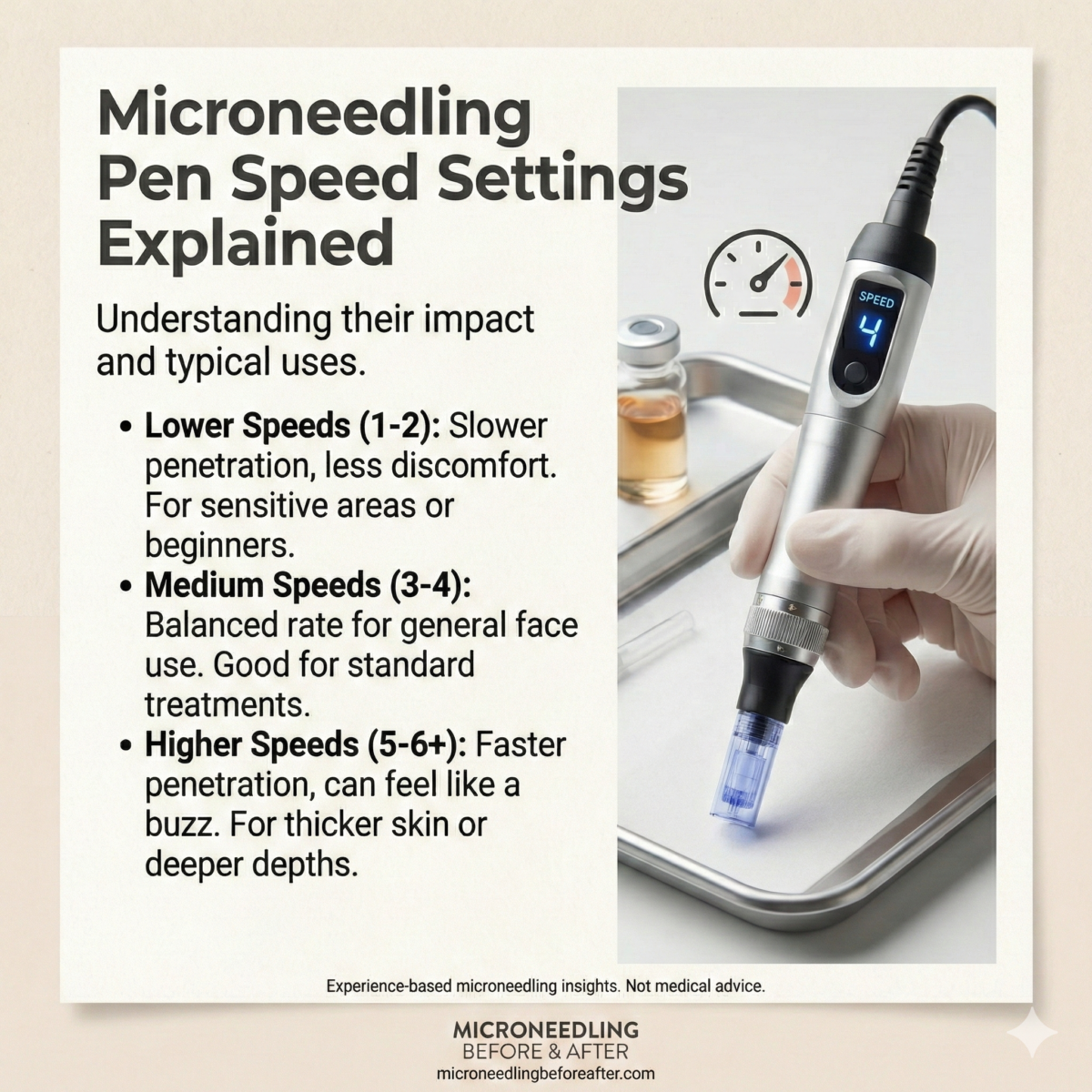
Speed settings explained: typical ranges and effects
I describe general speed categories (low, medium, high), provide a practical table mapping speed ranges to clinical indications, and explain how to interpret these settings in practice.
I include an evidence-informed table to help select speeds based on needle depth, anatomical area, and treatment intent.
| Speed category | Representative setting* | Approx punctures per second | Typical needle depth range (mm) | Clinical uses | Pros | Cons |
|---|---|---|---|---|---|---|
| Low | 1–3 | 20–60 | 0.25–2.5 (deeper treatments) | Deep scar remodeling, stretch marks, thicker tissue (back of neck) | More controlled entry, less tearing, better for deeper depths | Longer session time, more operator fatigue |
| Medium | 4–6 | 60–120 | 0.25–2.0 | General face, neck, moderate scarring, combined PRP | Balance of speed and control, efficient coverage | Moderate discomfort, careful technique needed |
| High | 7–12 | 120–200+ | 0.25–1.5 (shallower treatments) | Superficial rejuvenation, periocular (very shallow), fast sessions | Fast coverage, less time in clinic | Increased friction, potential microtearing if depth too deep |
*Representative setting values vary by manufacturer and model. I recommend referring to device-specific manuals for precise units.
I point out that manufacturers may label settings numerically; those numbers are not standardized. I therefore translate them qualitatively for practical use.
Interpreting punctures per second
I explain that punctures per second equals needle count × strokes per second. For example, a 12-needle cartridge at 100 strokes per second yields 1,200 punctures per second across the array, but the number of punctures per individual skin point depends on pass frequency.
I stress that more punctures per unit time can increase biological signaling but also increase transient inflammatory burden.
How speed interacts with needle depth and tissue type
I discuss how speed should be adjusted depending on depth and the anatomical area. I provide a second table with recommended speed-depth pairings as a starting point.
| Area / Concern | Typical needle depth (mm) | Recommended speed category | Rationale |
|---|---|---|---|
| Periorbital (under-eye) | 0.2–0.5 | Low to Medium | Thin skin requires shallow depth and careful speed to avoid bruising and hematoma |
| Full face rejuvenation | 0.5–1.5 | Medium | Balance of coverage and comfort |
| Acne scars (rolling/boxcar) | 1.5–2.5 | Low to Medium | Deeper depths for dermal remodeling; slower speeds reduce tearing |
| Stretch marks / body | 1.5–3.0 | Low | Thick tissue requires deeper penetration and careful pass control |
| Scalp for hair growth | 0.5–2.0 | Low to Medium | Needle depth varies by follicle depth; slower speeds for deeper scalp entry |
I caution that these are starting points and must be individualized based on patient comfort, skin thickness, and any prior treatments.
Why deeper treatments favor lower speeds
I explain that at greater depths, the needle engages more fibrous dermal tissue which resists entry. Lower speeds reduce lateral shear and the “piston” effect that can tear tissue rather than creating clean microchannels.
I add that slower speeds at depth also allow better control of needle alignment and decrease the risk of bleeding and prolonged downtime.
Practical selection of speed during a session
I outline a step-by-step framework I use: assessment, test area, progressive titration, and documentation.
I provide specific tips for clinicians and responsible home users.
Assessment and test patch
I always assess skin thickness, scar type, vascularity, and pain tolerance before selecting speed. Then I perform a small test patch at the planned depth and a medium speed to observe tissue response.
I recommend checking for pinpoint bleeding, excessive erythema, or bruising. Based on the response, I adjust speed up or down.
Progressive titration across zones
I explain that I often use variable speeds within a single session: slower speeds for cheeks with deep scars, medium speeds for forehead, and faster speeds for superficial textural concerns.
I emphasize documentation of settings for reproducibility and follow-up comparisons.
Patient communication and pain control
I advise telling patients what sensations to expect and using topical anesthetic appropriately when treating deeper depths. I recommend slower speeds if the patient reports excessive discomfort.
I also describe techniques to reduce pain: consistent pressure, short bursts rather than continuous high-speed passes, and adequate numbing when indicated.
Combining microneedling with adjunctive therapies
I discuss how speed influences synergistic use of PRP, topical peptides, vitamin C, and tranexamic acid, and provide guidance on safety and timing.
I note that adjuncts change the risk profile and therefore speed considerations.
PRP and growth factors
I explain that PRP applied immediately after needling benefits from open microchannels, but high-speed treatments could generate more bleeding that dilutes the PRP on the surface.
I recommend moderate speeds when combining with PRP at deeper depths to balance channel formation and retention of PRP at the dermal interface.
Topical actives and serums
I caution that needles enable higher penetration of actives and that some ingredients (e.g., retinoids, acids) can irritate subepidermal tissue if applied immediately after needling.
I suggest using sterile, balanced serums specifically formulated for post-needling use, and adjusting speed to avoid excessive systemic absorption or irritation.
Safety, contraindications, and infection control
I provide detailed safety guidance and contraindications and emphasize that speed influences tissue trauma and infection risk.
I include specific procedural controls I implement in practice to minimize complications.
Common contraindications
I list absolute and relative contraindications: active infection (HSV, bacterial), isotretinoin use within past 6–12 months, active acne cysts, uncontrolled diabetes, anticoagulation or bleeding disorders, keloid tendency, pregnancy in some practices, and unrealistic expectations.
I advise postponing treatment or selecting superficial depths and slower speeds in borderline cases, but often highest caution is to avoid needling until contraindications are resolved.
Sterility and cross-contamination prevention
I describe single-use sterile cartridges, skin antisepsis (e.g., chlorhexidine or alcohol), and proper glove use. I explain that higher speeds may aerosolize fluids slightly more, so good barrier precautions and minimal spraying of serums reduce contamination risk.
I also advise against needling through non-sterile cosmetics and recommend a clean environment and proper disposal.
Managing adverse events
I outline common side effects — transient erythema, edema, pinpoint bleeding, bruising, and minimal crusting — and more serious complications such as infection, hyperpigmentation, and scarring.
I explain that adjusting speed downward in subsequent sessions often helps reduce repetitive trauma and allows tissue recovery.
Aftercare and recovery timeline
I provide a practical aftercare protocol and expected timeline for visible results, emphasizing how speed and depth influence recovery.
I present a concise table summarizing expected immediate and delayed reactions.
| Timeframe | Typical reactions | Care recommendations |
|---|---|---|
| Immediately (0–24 hours) | Erythema, mild swelling, pinpoint bleeding | Cold compresses, gentle cleanser, no makeup, sterile serums if indicated |
| 24–72 hours | Peeling, persistent redness in deeper treatments | Hydrating occlusives, sunscreen, avoid exfoliants and active acids |
| 3–7 days | Skin texture improving, residual redness | Resume gentle skincare, monitor for infection |
| 2–12 weeks | Collagen remodeling begins, visible improvement | Maintain sunscreen, consider maintenance sessions |
I stress that higher speed superficial treatments often have quicker normalization, while deeper low-speed treatments have longer redness but potentially greater long-term remodeling.
Frequency of treatments and maintenance
I recommend a series of 3–6 sessions spaced 4–8 weeks apart for most indications, with the interval adjusted by treatment depth and patient recovery.
I advise maintenance sessions every 6–12 months after the initial series depending on goals and skin response.
Clinical evidence and studies
I summarize the evidence base supporting microneedling’s efficacy for scars, photoaging, melasma, and hair loss, noting how treatment parameters influence outcomes.
I emphasize that while many studies exist, protocols are heterogeneous and speed is often underreported.
Efficacy for scars and photoaging
I note randomized and observational studies that demonstrate improvement in acne scarring and skin texture with microneedling, particularly when combined with PRP or topical growth factors.
I point out that protocols using deeper needle lengths and controlled lower speeds for scar remodeling tend to show more robust dermal improvements.
Melasma and pigmentary disorders
I explain that microneedling can enhance depigmenting agent delivery and may improve stubborn pigmentary conditions. However, careful parameter selection is required because excessive trauma can exacerbate post-inflammatory hyperpigmentation (PIH).
I recommend lower speeds with superficial depths for patients prone to PIH, and prudent use of adjunctive depigmenting agents.
Troubleshooting common issues
I provide practical solutions to common challenges: inconsistent penetration, excessive bleeding, hyperpigmentation, and patient pain.
Each problem includes a protocol for adjusting speed and other parameters.
Inconsistent penetration or “skipping”
If I notice cartridges skipping across the skin, I first check tissue tension and hand positioning. Increasing tissue tautness and reducing speed often correct the issue.
I also consider cartridge wear or dull needles as causes and replace cartridges accordingly.
Excessive bleeding or bruising
I reduce needle depth and speed on subsequent passes, apply pressure to stop bleeding, and consider temporary discontinuation of anticoagulants in coordination with the patient’s physician.
I assess for underlying bleeding disorders if bleeding is disproportionate.
Post-inflammatory hyperpigmentation (PIH)
I slow down speeds, reduce depth, and incorporate pre- and post-treatment topical lighteners as appropriate. I also advise strict photoprotection.
I monitor skin response and postpone further sessions until pigment stabilizes.
Practical tips for clinicians and home users
I outline my rules of thumb for safe, effective practice, including documentation and patient education.
I include checklists and brief protocols for common scenarios.
Clinician checklist before treatment
- Conduct a full medical history and skin assessment.
- Determine needle depth and speed plan by zone.
- Perform test patch and document response.
- Use sterile single-use cartridges and proper antisepsis.
- Provide patient with written aftercare instructions.
I stress the importance of documenting speed, depth, passes, and adjuncts used for reproducibility.
Home-use device considerations
I caution that home devices typically use shorter needles (≤0.3–0.5 mm) and lower speeds, and that users should follow manufacturer guidance.
I recommend that home users avoid high-speed devices with long needles, and consult a professional for deeper treatments.
Ethical and regulatory considerations
I discuss licensing and scope of practice issues, and the need to follow manufacturer instructions and local regulations.
I make clear that speed adjustments are a clinical parameter that should be governed by training and oversight.
Training and competence
I require formal training for any clinician performing microneedling and recommend supervised practice for several procedures before independent practice.
I believe that understanding device mechanics, sterility, speed-depth interactions, and complication management is essential.
Informed consent
I always obtain informed consent that includes discussion of the role of speed and depth, expected outcomes, alternatives including topical-only regimens, and risks.
I document the discussion and the agreed-upon parameter plan.
Conclusion
I have explained microneedling pen speed settings in the context of device mechanics, biological rationale, clinical selection, safety, and evidence. I emphasized that speed is not an isolated variable — it must be chosen in concert with needle depth, cartridge type, tissue characteristics, and adjunctive therapies.
I recommend that clinicians individualize settings using test patches and progressive titration, document everything, and prioritize patient safety. Patients should understand why microneedling can work when skincare alone fails and seek qualified providers for deeper or more aggressive treatments.
If you would like, I can provide a printable quick-reference table of speed-depth recommendations tailored to a specific device model or a sample informed consent and documentation template that includes speed settings.