? Am I putting my thin skin at risk if I try microneedling, or can I safely get the benefits without compromising my skin barrier?
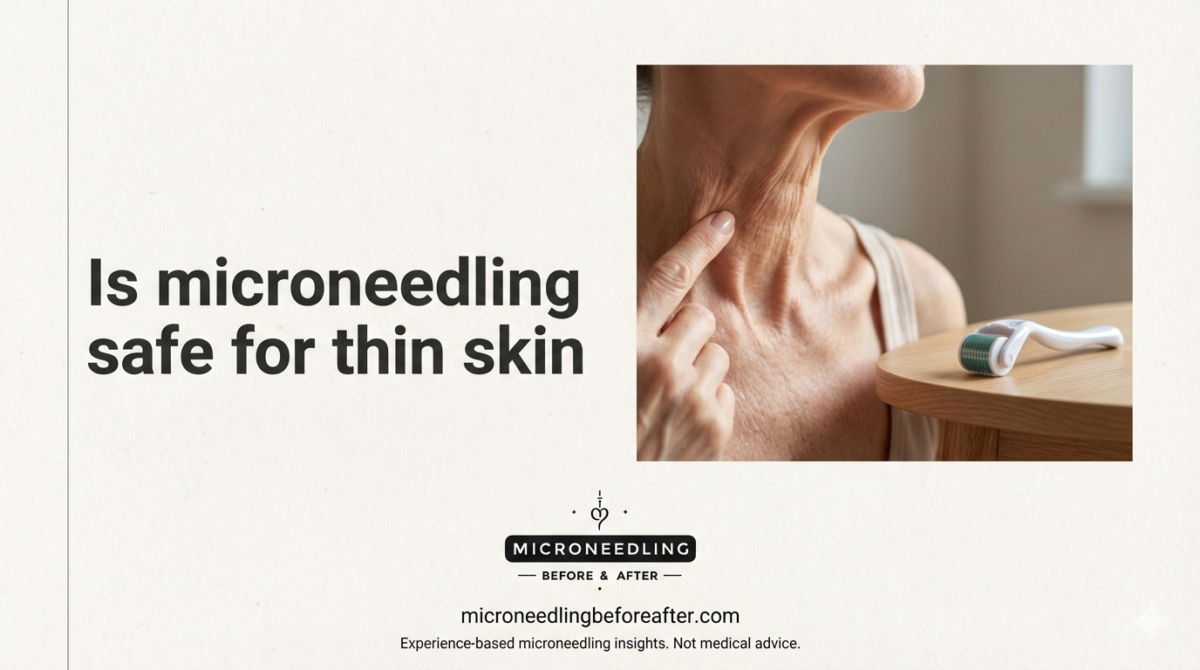
Is Microneedling Safe For Thin Skin
I get asked this question a lot, and I want to walk through it carefully so you can make an informed decision. I’ll explain what microneedling does, why thin skin matters, and how to make a safe plan if you decide to proceed.
What is microneedling?
Microneedling is a skin procedure that uses tiny needles to create controlled micro-injuries in the skin. I think of it as a way to stimulate the skin’s natural repair response—collagen and elastin production—rather than an aggressive resurfacing.
How microneedling works
When the needles puncture the skin, the controlled trauma triggers inflammation followed by tissue remodeling and collagen synthesis. I find it helpful to remember that the goal is controlled repair: the needles are shallow enough to avoid major damage but deep enough to kickstart healing mechanisms.
What I mean by “thin skin”
When I refer to thin skin, I mean skin with decreased dermal thickness, often with increased translucency, visible blood vessels, fine wrinkles, and a fragile-looking texture. Thin skin can be a natural trait, the result of sun damage, age-related collagen loss, long-term topical steroid use, or certain medical conditions.
Why thin skin matters for microneedling
Thin skin has less cushion and fewer structural proteins, so it may respond differently and be more easily injured by mechanical procedures. I treat thin-skin cases with extra caution because the risk of prolonged redness, bruising, or even atrophic scarring can be higher.
Benefits of microneedling for thin skin
Microneedling’s main benefits—stimulating collagen and improving texture—can still apply to thin skin, and many people with thin skin report smoother, firmer results over a course of treatments. I’ve seen thin-skin clients benefit, especially when microneedling is adapted (shallower needle depths, longer intervals) and combined with protective aftercare.
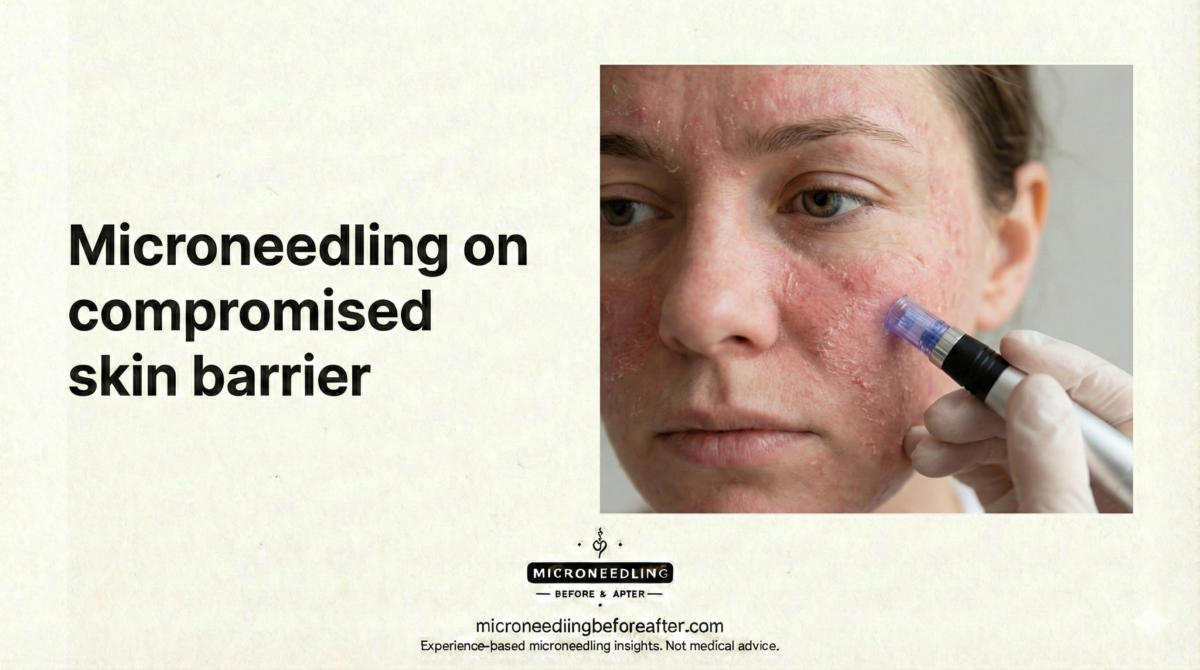
Risks and complications specific to thin skin
Risks I worry most about are prolonged erythema (redness), purpura (bruising), persistent sensitivity, broken capillaries, and in rare cases atrophic scarring or hyperpigmentation. I also watch for poor wound healing in people with underlying conditions, and I emphasize that even minor infection risk is more consequential when the barrier is fragile.
Typical complications and what they look like
Minor complications include redness, pinpoint bleeding, swelling, and temporary sensitivity that usually resolves in days. Major or persistent complications—scarring, prolonged hyperpigmentation, or significant infection—are uncommon but can be more problematic on thin skin if not managed promptly.
How skin thickness is measured
Dermatologists or providers often estimate skin thickness clinically by inspection and palpation, and more precise measurement can be done using ultrasound or high-resolution imaging. I recommend an objective assessment when the skin looks unusually thin or when steroid use or other risk factors are present.
Causes of thin skin I check for
I always ask about long-term topical or systemic steroid use, significant sun exposure, aging, medical history (like connective tissue disorders), and history of cosmetic procedures. I find that addressing reversible causes and optimizing skin health before microneedling lowers the chance of complications.
Who should avoid microneedling
I advise people to postpone or avoid microneedling if they have active skin infections, severe acne, keloid tendency, uncontrolled diabetes, recent isotretinoin use (within 6–12 months in many protocols), or untreated clotting disorders. If I suspect thin skin secondary to steroid misuse or an underlying systemic issue, I want those factors managed first.
Professional microneedling vs at-home devices
I prefer professional microneedling for people with thin skin because professionals can adjust needle depth, technique, and use sterile protocols that lower complication risks. At-home rollers and pens generally have shallower needles but carry infection and technique risks; I’m cautious about recommending them for fragile skin.
Table: Professional vs At-Home Microneedling — Considerations for Thin Skin
| Feature | Professional Microneedling | At-Home Derma-Rollers / Pens |
|---|---|---|
| Needle depth control | Precise, adjustable (0.25–3.0 mm) | Typically shallower, variable control |
| Sterility | Performed under sterile conditions | Higher contamination risk |
| Provider assessment | Pre-screening and tailored protocol | No professional assessment |
| Pain control | Topical anesthesia optional | Limited pain control |
| Post-care supervision | Professional follow-up | Self-managed |
| Safety for thin skin | Safer when adjusted by pro | Higher risk if misused |
Needle depth and settings — recommendations for thin skin
For thin skin, I recommend conservative needle depths, generally between 0.25 mm and 0.5 mm for cosmetic improvement without deep dermal trauma. If a provider considers deeper treatment (0.75–1.5 mm), I expect clear justification and a very careful patient selection process—sometimes starting with test spots.
How many sessions and spacing I usually recommend
I often suggest an initial series of 3–6 sessions spaced 4–8 weeks apart, depending on response and tolerance. For thin skin, I tend to increase the interval between sessions to allow full recovery and to monitor for delayed adverse reactions.
Pre-treatment assessment and testing I recommend
Before I agree to microneedling, I take a full medical and medication history, assess skin condition, look for signs of thinning, and sometimes request a dermatologist consultation or ultrasound in uncertain cases. If recent isotretinoin use or systemic steroids are present, I delay treatment according to safety guidelines.
Preparing the skin before microneedling
I advise improving skin barrier function pre-procedure: stop irritant actives (like retinoids and exfoliants) for a week or more, maintain hydration with gentle moisturizers, and use sun protection. I sometimes recommend topical growth-factor–free serums and barrier-supporting ingredients, but I avoid introducing too many new products right before a session.
The procedure: what happens during a session
During a professional session the provider will cleanse the skin, apply topical numbing if needed, and pass a sterile needle device across the treatment area in controlled passes. I remind people that slight pinpoint bleeding and fresh redness are expected and that the provider should use gentle pressure and appropriate settings for thin skin.
Pain, anesthesia, and comfort measures
I tend to use topical anesthetic for comfort when deeper settings are required, and I explain that shallower treatments may only cause minor stinging. I also suggest stress-relieving measures like breathwork and distraction because less anxiety can improve tolerance and reduce involuntary movement.
Aftercare and recovery for thin skin
My aftercare recommendations emphasize protecting the compromised barrier: gentle cleansing, fragrance-free moisturizers, and broad-spectrum sunscreen starting immediately when the skin is no longer weeping. I counsel patients to avoid exfoliants, active ingredients (retinoids, acids), saunas, and vigorous exercise for several days to limit irritation and infection risk.
Typical recovery timeline I discuss
In most cases, redness and tightness improve within 48–72 hours, with continued improvement over a week; I tell patients to expect some change in texture and mild flaking for up to two weeks. For thin skin, I monitor for longer-lasting redness or sensitivity and adjust subsequent session timing accordingly.
Managing complications if they occur
If I see signs of infection—increasing pain, pus, fever—I instruct immediate medical attention and usually start appropriate antibiotics. For prolonged redness, bruising, or post-inflammatory hyperpigmentation, I consider topical anti-inflammatories, light-based therapies, and pigment-directed treatments once the skin barrier is adequate.
Table: Common Complications and My Typical Interventions
| Complication | Timeframe | Intervention I Recommend |
|---|---|---|
| Prolonged redness | >7–14 days | Anti-inflammatory topicals, cooling, extended observation |
| Bruising/purpura | 1–2 weeks | Cold compresses initially; time and avoidance of blood thinners |
| Infection | Any time post-procedure | Urgent evaluation; topical/systemic antibiotics as needed |
| Hyperpigmentation | Weeks to months | Sun protection, topical bleaching agents, or light therapy |
| Scarring | Weeks–months | Early referral to dermatology; intralesional steroids for hypertrophic scars |
At-home microneedling: is it worth the risk for thin skin?
I rarely recommend at-home microneedling tools for thin skin because of the variability in needle quality, hygiene, and technique. If someone insists, I require strict instructions—safe needle length (≤0.25–0.3 mm), rigorous sterilization, and immediate cessation if there’s excessive redness or bleeding.
Combining microneedling with PRP or topical serums
I’ve seen microneedling paired with platelet-rich plasma (PRP) or topical serums to potentially enhance healing and collagen stimulation, but I’m cautious combining active biologicals on thin skin without clear safety data. If PRP is used, I want it prepared and applied under sterile conditions and at shallow depths; for topical serums, I choose those that support barrier repair rather than increase inflammation.
Timing after other cosmetic procedures
I delay microneedling after lasers, chemical peels, or surgical procedures until the skin has fully healed. I also wait appropriate intervals after filler injections or neuromodulator treatments unless the provider is experienced in combining modalities safely.
Alternatives for thin skin that I consider
When microneedling seems risky, I consider gentler collagen-stimulating options: low-strength chemical peels, topical peptides, prescription retinoids in controlled doses, light therapies (LED, low-energy lasers), and cosmeceuticals to rebuild the dermal matrix. I also sometimes recommend collagen-boosting injectables or biostimulatory fillers done very conservatively.
Table: Alternatives to Microneedling — Pros and Cons for Thin Skin
| Treatment | Pros | Cons |
|---|---|---|
| LED light therapy | Non-invasive, reduces inflammation, supports healing | Slower, milder results |
| Low-strength chemical peels | Can improve texture with controlled exfoliation | Risk of irritation if skin is very thin |
| Topical retinoids (low dose) | Stimulate collagen with careful use | Can initially irritate and thin barrier if misused |
| PRP alone (no microneedling) | Autologous growth factors, lower mechanical trauma | Variable evidence, procedural requirements |
| Biostimulatory injectables (very conservative) | Stimulate collagen without epidermal puncture | Risk of nodules, requires experienced injector |
Combining microneedling with other therapies: timing and safety
If I plan to combine treatments, I schedule microneedling at least several weeks away from thermal or ablative procedures and consult with the other treating clinicians. Careful timing reduces overlapping inflammation and minimizes cumulative risk to thin skin.
How I choose a provider for microneedling
I look for providers with medical training—dermatologists, physician assistants, or experienced nurses—who show knowledge about skin thickness, have strict sterile practices, and tailor protocols. I also value before-and-after photos of similar skin types and clear explanations of contingency plans for complications.
Questions I always ask my provider
I ask about their sterilization protocols, specific needle depths they plan to use, how they assess thin skin, the exact post-care regimen, and whether they will perform a test spot if I have risk factors. I also ask about emergency procedures and follow-up scheduling.
Cost considerations and value
Costs vary widely depending on location, provider credentials, and whether adjuncts like PRP are included; I usually weigh price against provider expertise rather than looking for the cheapest option. For thin skin, I’m willing to pay more for an experienced clinician because careful technique and follow-up reduce downstream costs and complications.
Realistic expectations I set for results
I tell people that microneedling can improve fine lines, texture, and superficial scarring, but results are gradual and depend on skin biology and adherence to aftercare. For thin skin, I emphasize modest goals: improved texture and tone without aggressive changes that could compromise the barrier.
When to stop or pause treatment
If I encounter persistent redness, repeated delayed healing, or spreading broken capillaries after a session, I pause further treatments and reassess. I also stop if there’s an acute medical issue that could impair healing, like new systemic steroid use or uncontrolled blood sugar.
My practical checklist before booking a session
I make sure I’ve disclosed all medical history, stopped irritant topicals in time, confirmed my provider’s credentials and sterile practice, and arranged for sensible aftercare (time off, gentle products). I also plan to document my skin’s baseline condition with photos to track changes.
Final verdict: Is microneedling safe for thin skin?
I believe microneedling can be safe for thin skin when done conservatively by experienced providers who tailor needle depth, spacing, and aftercare to individual risk. However, I also recognize that thin skin increases sensitivity to complications, so careful assessment, conservative settings, and strict post-procedure care are essential.
Frequently Asked Questions
Can microneedling make thin skin worse?
Yes—if performed too aggressively or without consideration for thin-skin characteristics, microneedling can prolong redness, cause bruising, or in rare cases lead to scarring. I stress gentle protocols and staged treatment to minimize that risk.
Can microneedling cause permanent damage to thin skin?
Permanent damage is uncommon but possible if there’s infection, poor healing, or inappropriate depth and frequency. I reduce that risk by screening for contraindications and monitoring healing closely.
Is microneedling with PRP safe for thin skin?
PRP may enhance healing, but combining microneedling with PRP on thin skin should be done by clinicians experienced with both procedures. I want PRP applied under sterile conditions and would likely use shallower depths to reduce mechanical trauma.
Is at-home derma rolling safe if my skin is thin?
I generally do not recommend at-home derma rollers for thin skin because of hygiene, uncontrolled pressure, and variable needle quality. If someone insists, I set strict limits: very shallow needles, impeccable sterilization, and immediate cessation for excessive redness.
How long before I see improvements?
Some textural improvement may appear within weeks, but most people notice meaningful results after a series of 3–6 sessions over several months. I encourage patience and consistent aftercare to let collagen remodeling occur.
Will microneedling reduce thinness or actually thicken my skin?
Microneedling stimulates collagen production and can modestly increase dermal thickness over time, but it won’t restore dramatically lost tissue in a single session. I recommend realistic goals—incremental improvement rather than full reversal of severe thinning.
What if I’m on topical steroids or had recent steroid use?
Topical and systemic steroids can thin skin and impair healing; I prefer a steroid-free interval and sometimes dermatology consultation before proceeding. If steroid therapy is ongoing for a medical reason, I coordinate with the prescribing clinician.
How should I protect my skin immediately after treatment?
I use gentle cleansing, a bland moisturizer, and physical sunscreen once skin has stopped oozing; I avoid active ingredients until the skin is fully healed. I also avoid makeup for at least 24–48 hours, depending on provider instructions.
Can microneedling help with visible blood vessels and translucency?
Microneedling may reduce the appearance of fine lines and improve texture, but visible blood vessels are often better treated with vascular-targeted therapies like pulsed dye laser or IPL. I sometimes combine approaches, but vascular treatments must be chosen carefully for thin skin.
When should I seek medical attention after microneedling?
I seek urgent care if I see spreading redness, increasing pain, pus, fever, or signs of systemic infection. For persistent redness, hyperpigmentation, or scarring, I consult dermatology for targeted interventions.
I hope this gives you a clear, practical framework to decide whether microneedling is right for thin skin. If you want, I can help you prepare a list of questions to bring to a consultation or walk you through an individualized risk checklist based on your medical history and skin exam.